Abstract
Purpose
Glioblastoma is a malignant brain tumor which has one of the poorest prognosis. It is not clear if toxic environmental factors can influence its aggressiveness. Recently, it was suggested that brain cancer patients with heavy cell phone use showed reduced survival. Here we aimed to assess the effect of controlled brain averaged specific absorption rate (BASAR) from heavy use of cell phone radiofrequency electromagnetic fields (RF–EMF) on in vivo C6 brain tumors in Wistar rats.
Methods
C6 cells grafted male rats were exposed to GSM 900 MHz signal at environmental BASAR, 0 (sham), 0.25 or 0.5 W/kg (5 days a week, 45 min a day in restraint), or were cage controls (no restraint). At death, tumor volume and immunohistochemistry for CD31, cleaved caspase (CC) 3 and Ki67 were assessed to examine vascularization, apoptosis and cellular divisions, respectively. Moreover, immune cell invasion, necrosis and mitotic index were determined.
Results
Results showed no BASAR effect on survival (31 days post-graft median), tumor volume, mitotic index, vascularization, infiltration, necrosis or cell division. However, results suggested a BASAR-dependent reduction of immune cell invasion and apoptosis.
Conclusions
Our data suggested an action of RF–EMF by reducing immune cell invasion and glioblastoma cell apoptosis, at probably too low amplitude to impact survival. Further replication studies are needed to confirm these observations.
Similar content being viewed by others
Introduction
Glioblastoma multiforme (GBM), the most common primary malignant brain tumor, has features of rapid and invasive growth in the brain with an extremely poor prognosis [1, 2]. Meanwhile, the use of mobile phones has increased rapidly since the early 1980s and the health effects of radiofrequency electromagnetic field (RF–EMF) exposures are a growing public concern regarding brain cancer. RF–EMF were classified as a possible carcinogen for humans (risk group 2B) by the International Agency for Research on Cancer (IARC) [3]. Several epidemiological studies suggested possible effects of intensive cell phone use on brain cancer incidence [3,4,5,6]. In patients with GBM, high cumulative cerebral cell phone exposures were suggested to be linked with survival disadvantage or benefit in grade IV or I–II, respectively [7]. However, neither causal relationship nor established biological or biophysical mechanism of action were demonstrated using controlled RF–EMF exposures in animal experiments. In the RG2 glioma rat model, tumor size was not impacted after daily continuous or modulated 915 MHz microwaves [8]. Another study didn’t report any effect of daily RF–EMF exposures on survival in the gliosarcoma (9L cells) rat model using the frequency-modulated continuous 835.62 MHz wave or code division multiple access centered on 847.74 MHz [9].
A recent systematic analysis of in vitro studies suggested no causal relationship between RF–EMF and cellular life (for review see [10]). Cleaved caspase (CC)3 is a determinant of the onset of cellular apoptosis and its decreased activated expression in patients was linked to lower survival [11]. In vitro, astrocytes showed increased CC3 to 48 h RF–EMF exposures while apoptosis, proliferation, doubling time or growing curve of C6 GBM cells were not shown to be affected by RF–EMF exposures [12]. RF–EMF was suggested to stimulate C6 cell thymidine incorporation at a specific absorption rate (SAR) of 59 µW/g [13]. In vivo, only a few studies were performed. Dasdag and collaborators reported decreased brain CC3 in glial cells in Wistar rats daily exposed for 10 months to the Global System for Mobile Communications (GSM) signal with a 0.17–0.58 W/kg SAR [14].
Cellular proliferation is highly increased in GBM. One of its biomarker, the Ki-67 protein was suggested to predict aggressiveness and to have a prognostic value in astrocytic gliomas [15]. In vitro, proliferation and mitotic index of various cell types were not modified after RF–EMF exposures [16,17,18]. In vivo, a normal proliferation ratio was shown on skin cells using Ki-67 after acute or chronic GSM 900 or 1800 MHz RF–EMF exposures [19, 20].
In direct relation with tumor growth, vascular patterns of brain tumors play important roles in prognostic [21,22,23,24]. GBMs show extreme levels of microvascular hyperplasia. Tepper et al. showed that pulsed EMF increased angiogenesis [25]. However, there is no published data in response to the GSM signal.
Immune cell invasion plays an important role in neural physiopathology. RF–EMF were shown to affect inflammation in healthy rat brains involving microglia and astrocytes activation [26,27,28]. Various inflammatory cytokines promote the growth, survival, and invasion of GBM cells [29].
Important public health consequences may be anticipated if the large environmental exposure to RF–EMF increased brain tumor progression in heavy cell phone users. Here we aimed to assess the effect of controlled brain averaged (BA)SAR from heavy use of cell phone RF–EMF on in vivo C6 brain tumors in Wistar rats.
To that end, a human-like GBM C6 cell rat model was locally exposed to the brain with RF–EMF until death [30]. Brain averaged specific absorption rate (BASAR) were ICNIRP-compatible local limits to the brain for human exposure. Tumor size, CD31, CC3 and Ki67 immunohistochemistry as well as immune cell invasion, necrosis, infiltration and mitotic index were assessed at death.
Materials and methods
Animals
At postnatal day (PND) 30, 201 male Wistar rats (Janvier Lab, France) were housed in a controlled environment (room temperature 22 °C, 12 h light/dark cycle, food and water ad libitum) and acclimatized for 5 days before the surgery. After surgery, the rats were housed four per cage in enriched environment with plastic cylinders similar in shape to RF–EMF exposure rockets. Protocols complied with the decree on vertebrate animal experiments (French State Council, 1987) and were approved by the Regional Ethical Committee N. 96 (CREMEAP).
Experimental groups
Rats were grafted and randomly assigned to the sham (0 W/kg), 0.25 W/kg, 0.5 W/kg or to the cage control (CC, no restraint) groups. RF–EMF exposures were performed in restraint for 45 min per day, 5 days per week, starting on post-surgical day (PSD)7 and ending at death or at PSD65.
RF–EMF exposures
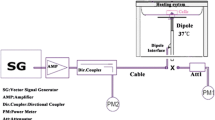
A RF power source (900-64 type, generator RFPA S.A., RFS9001800-25 model, RF Power Amplifier, France) emitting a GSM 900 MHz EMF (1/8 duty factor) pulse modulated at 217 Hz was connected to a four-way divider. Each output was connected to a loop antenna [31] that consisted of a two-metallic line circuitry printed on a dielectric substrate, which allowed four simultaneous head-exposures in the two exposure Faraday cages and one sham Faraday cage. A RF detector controlled incident and reflected powers. Exposures were performed daily for 45 min between 9:00 a.m. and 1:00 p.m. in a blinded fashion.
Exposure duration aimed to mimic the heavy user’s category. Based on questionnaires of previous epidemiological studies it was included in the interval between the regular (1 call per week) and the extremely frequent (5 h per day) cell phone user categories [5]. According to our previous studies, repeatedly 45 min restrained rats elicited cage control-like stress and behaviors [26]. The rat body was restrained in cylinders (4.5, 5 and 6 cm in diameter to adapt to rat body size growth between PSD7 and PSD65) holed to minimize body temperature elevation. The head was inserted at restrainer end in a truncated cone to allow the animal to breathe. According to the finite difference time domain calculations, numerical dosimetry indicated that input powers of 0.17 and 0.33 W gave BASARs of 0.25 or 0.5 W/kg, respectively.
C6 cells preparation and tumor grafting
The C6 rodent models of GBM was shown to recapitulate features of human GBM [30, 32]. C6 cells were purchased from ATCC (C6ATCC®CCL-107™, LGC Standards, Molsheim, France). They were grown in F-12K (Kaighn’s Modification of Ham’s F-12) Medium, supplemented with 2.5% fetal bovine serum and horse serum to a concentration of 15%, at 37 °C in 5% carbon dioxide at 90% relative humidity. They were seeded at a concentration of 500,000 cells/flask. Once arrived at 80% confluence, cells were suspended in complete PBS buffer (with calcium and magnesium) at a concentration of 1.12 × 106 cells/ml and kept on ice prior to the graft. Injection of 5600 C6 cells were performed in 5 µl in anesthetized rats placed in the stereotaxic apparatus. The 26 Gauge needle (ga26/51 mm/pst3, Phymep SARL, France) was inserted in the striatum (+ 0.96 mm antero-posterior to the bregma, − 3 mm lateral, − 6 mm dorso-ventral) [33]. At the end of the injection, a 10 min’ time lapse was awaited before gently raising the syringe to prevent the cells from sticking-out along the needle.
Monitoring and pathology
We explored the outcome survival. Monitoring was performed in a blinded fashion according to Higashikubo et al. [9]. Briefly, rats were examined twice daily for the development of clinical/neurological and were daily weighed. Weight loss, progressive deterioration, and increase in the number of neurological signs that indicate imminent death were used to select animals for euthanasia. All survivors were euthanized at PSD65.
Anatomopathology
We explored the outcomes tumor size and categorized tumor location by dividing the brain into seven structures. Rats were anaesthetized with isoflurane and received intra-cardiac PBS followed by formaldehyde 4% infusions. Brains were extracted, fixed in 4% formaldehyde for 5 days and stored in ethanol. Using a brain matrix, frontal sections were made at the optical chiasma (0 mm), + 3 mm, + 6 mm and + 9 mm. For each animal, the 3–5 sections containing tumorous tissue were dehydrated using the Vacuum Infiltration Processor (VIP 5, Sakura) and embedded in paraffin wax. Blocks were sectioned using the Microtome Tissue Tek® Accu-Cut® SRM 200 (Sakura). Slides were stained using the DS 2000 from Sakura or immune-labelled using the DAKO link 48. The sections were mounted on Tissue-Tek Coverslipper SCA 5600 (Sakura) and stained with hematoxylin and eosine (HE). All pathological examinations were performed in a blinded fashion. Numerical images of the slides were produced using the Nanozoomer camera from Hamamatsu at X20 magnification. Tumor length (l), wide (w) and height (h) were measured. The tumor volume was calculated using the formula:
Immunohistochemistry for CC3, Ki67 and CD31
We explored the following outcomes: apoptosis, cell division and vascularization as measured, respectively by caspase 3 (CC3), Ki67 and CD31 immunoreactivity, immune cell invasion and necrosis as measured on a scale from 1 to 5.
Paraffin sections were deparaffinized and antigen retrieval was carried out with the Dako Target Retrieval Solution. The protocol for retrieval included 20 min at 98 °C in EDTA pH9 for CC3, 20 min at 98 °C in citrate pH6 for Ki67, and 10 min at 121 °C in EDTA pH9 for CD31. Rabbit monoclonal antibody to CC3 (Cell signaling, #9664), rabbit polyclonal antibody to Ki67 (Abcam, ab15580) and to CD31 (Abcam, ab28364) were used as primary antibodies at the concentrations of 1/800, 1/1000 and 1/200, respectively. After blocking endogenous peroxydase activity (Dako EnVision®+ System-HRP (DAB), Peroxydase Block) and blocking nonspecific protein labeling (DAKO Protein block serum free), the slides were incubated with diluted primary antibody for 1 h at room temperature. The tissue sections were then washed and incubated with appropriate secondary antibodies for 30 min (Dako, K4011). Immuno-reactive signals were detected using DAB substrate solution (Dako EnVision®+ System-HRP, DAB + substrate buffer/liquid DAB + chromogen, 5 min’ incubation). Finally, the sections were lightly counterstained with Mayer’s hematoxylin. Negative controls were obtained by substitution of the primary antibodies with isotype control immunoglobulin (rabbit IgG, Dako, X0936) in the immunohistochemical staining procedure, with a concentration equivalent to the primary antibody. Automatic quantification was performed on numerical slides. The number of mitotic figures were counted (five fields at magnification × 400 = 0.11 mm2). The tumor micro-environment was assessed by the semi-quantitative gradation of the severity of immune cell invasion (by counting macrophages and lymphocytes) and necrosis. Semi-quantitative gradation included 6 grades: 0 = no lesion, 1 = negligible, 2 = weak, 3 = moderate, 4 = marked, 5 = severe. All histological examinations were performed in a blinded fashion.
Statistics
Survival data and means ± standard error of the mean were analyzed using Graph Pad Prism Statistics software. RF–EMF effects were tested by comparing the sham-, 0.25 W/kg- and 0.5 W/kg-exposed groups together, using the log-Rank test for trend for survival data, the Spearman correlation coefficient and partial coefficient (adjusted on survival) for quantitative data and the chi-squared test of a contingency table analysis of the binary outcomes. Restraint effect was tested by comparing the CC group with the sham-exposed group using the log-Rank, the Mann–Whitney test, and the Fisher’s exact test of a contingency table analysis. All hypothesis tests used a criterion level of α = 0.05 excepted for the test of RF–EMF effect on tumor location in seven different areas (tested using an adjusted criterion level of α = 0.006).
Results
Survival
Injection of 5600 C6 cells in 5 µl leaded to a graft efficiency rate of 51% and a 31-day median survival without any experimental group difference (data not shown). Log rank test for trend performed on survival data presented in Fig. 1 indicated no RF–EMF effect (Chi-squared = 0.1, p = 0.7). Log rank test indicated no restraint effect [HR = 0.7 (0.36–1.45), p = 0.36]. Median survivals were respectively 35, 44, 31 and 40 days for the sham-, 0.25 W/kg-, 0.5 W/kg-exposed groups and for the CC group.
Tumor size and location
Tumor volumes were not correlated to BASAR (Spearman coefficient: − 0.16, p = 0.14, and adjusted coefficient: − 0.18, p = 0.49, Fig. 2) and were not modified by restraint (p = 0.58). Figure 3 reported tumor cell location in the brain. Tumor location among the seven structures was not different between groups (p > 0.006).
Ratios of tumor location in five brain areas, the meninges and bones in the C6 GBM rat model. The sagittal brain section (− 3 mm lateral to the Bregma according to Paxinos and Watson stereotaxic atlas) indicates the brain areas by numbers. Striatum (4) was the tumor injection site. The GBM developed in the anterior brain, in the olfactory bulb (1) and the frontal cortex (2), in the ventral brain, in the basal part of the rhombencephalon (3), in the dorsal brain, in the corpus callosum (5) and the neocortex (6), and outside of the brain, in the meninges and bone. Rats were cage controls (CC, n = 15), or RF–EMF exposed at 0 W/kg (n = 31), 0.25 W/kg (n = 18) and 0.5 W/kg (n = 39)
Immune cell invasion, necrosis and vascularization
Data presented in Fig. 4a indicated that immune cell invasion was reduced with the BASAR (inverse correlation, Spearman coefficient: − 0.4, p = 0.00008 and adjusted coefficient: − 0.4, p = 0.00003). No effect of RF–EMF was obtained on necrosis or vascularization (p > 0.05, Fig. 4b, c). There was no restraint effect (p > 0.05).
Grades for a immune cell invasion, b necrosis, and c CD31 immunohistochemistry in the C6 GBM rat model subjected to RF–EMF exposures. At death, brain were collected and histochemical observations were performed on HE colored brain sections. Rats were cage controls (CC, n = 15), or RF–EMF exposed at 0 W/kg (n = 31), 0.25 W/kg (n = 18) and 0.5 W/kg (n = 39). Immune cell invasion was reduced by the RF–EMF exposure with a BASAR-dependent effect (*p < 0.05, ***p < 0.001)
Cell markers
According to data presented in Fig. 5a, tumor CC3 immunoreactivity was reduced with the BASAR after adjustment on survival (Spearman: − 0.2, p = 0.07 and inverse correlation with the partial coefficient: − 0.3, p = 0.005). Ki67 immunoreactivity and the proportion of mitotic cells were not different among the exposed-groups (p > 0.05). There was no restraint effect (p > 0.05).
a CC3, b Ki67 immunohistochemistry, and c mitotic index in the C6 GBM rat model subjected to RF–EMF exposures. At death, brain were collected and histochemical observations were performed on HE colored brain sections. Rats were cage controls (CC, n = 15), or RF–EMF exposed at 0 W/kg (n = 31), 0.25 W/kg (n = 18) and 0.5 W/kg (n = 39). CC3 immunohistochemistry was reduced by the RF–EMF exposure with a BASAR-dependent effect after adjustment on survival (*p < 0.05)
Discussion
For the first time, BASAR-dependent effects in C6 tumor cells were suggested in vivo. There was no indication of BASAR-related modification of survival or tumor size. Our data suggested reduced immune cell invasion in the tumor micro-environment without any sign of modified necrosis or vascularization. Our data suggested reduced C6 tumor cell apoptosis, without modified cell division or mitotic index.
This study assessed controlled BASAR induced by loop antennas-emitting RF–EMF. This system was known to be transposable for human head exposure during daily cell phone use [19]. In this study, BASARs were at the general population environmental levels. The 0.25 and 0.5 W/kg BASARs in the rat were equivalent to 1 and 2 W/kg in the human head outskirts (the limit for ICNIRP recommendation). Daily 45 min exposures aimed to mimic heavy cell phone users.
The Wistar rat model of intracerebrally grown C6 cells showed several aggressiveness characteristics of human GBM. Our data confirmed rapid death, low apoptosis, invasiveness, high cell division rate, foci of tumor necrosis, fast growing and neovascularization previously described in the literature [30, 32].
In the present study, all experimental groups showed similar tumor size and survival. Main length of tumor was 9 mm and survival mean was 44 days post graft. A previous study in the rat RG2 glioma showed no effect of repeated whole-body exposures with the 217 Hz modulated 915 MHz TEM signal on tumor size [8]. Another study in the 9L gliosarcoma rat model reported no effect of repeated RF–EMF exposures with the CDMA 847.74 MHz signal on survival [9]. In an epidemiological study, Hardell and Calberg’s data suggested a link between intensive and long-term cell phone use with decreased survival in GBM patients with a Hazard ratio of 1.3 [1.03–1.7], suggesting weak association [7]. In our study, very weak effects may have been missed due to low statistical power. Our data did not indicate any important BASAR impact on survival. Accordingly, a current epidemiological study does not suggest any survival disadvantage linked to cell phone use in glioma patients [34].
Tumor location impacts survival of GBM-diagnosed individuals [35]. In previous studies, growth and invasion of GBM cells were shown to be promoted by various inflammatory cytokines [30]. Here, BASAR-dependent reduction of immune cell invasion in the tumor micro-environment had no significant impact on the invasiveness of the tumor. Previous studies in healthy rats reported very divergent effects of RF–EMF exposure on inflammation in the brain [26, 36, 37] or no effect on in vivo astrogliosis induced by cerebral lipopolysaccharide infusion [37].
In patients, the decreased activated expression of CC3, the hallmark of apoptosis, was linked to lower survival [11]. Accordingly, our data indicated that CC3 immunohistochemistry and survival were significantly correlated (data not shown). Here, data suggested that RF–EMF may reduce apoptosis in glioblastoma with a BASAR-dependent effect. There was no impact on tumor size or survival, probably because the RF–EMF-induced BASAR-dependent decreased apoptosis was a too weak effect or it was only a chance finding. To the contrary, a previous study suggested CC3 increase in mice astrocyte cultures [12]. Data discrepancies may come from exposure parameters and in vivo versus in vitro approaches.
Here, data supported the absence of effect of RF–EMF on cell division. Accordingly, previous in vitro studies suggested normal growth curve and cell doubling time in RF–EMF-exposed C6 cells with the TDMA signal [13] or cell cycle in C6 cells and in human glioblastoma cell lines in response to the 3G signal [12].
Conclusion
Our data suggested an effect of RF–EMF by reducing immune cell invasion in the tumor micro-environment and by decreasing glioblastoma cell apoptosis. As no effect was seen on survival, the associations may have been too weak or have arisen by chance. Further replication studies are needed to confirm these observations.
References
Scott JN, Rewcastle NB, Brasher PM, Fulton D, MacKinnon JA, Hamilton M et al (1999) Which glioblastoma multiforme patient will become a long-term survivor? A population-based study. Ann Neurol 46(2):183–188
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359(5):492–507
IARC Monographs on the Evaluation of Carcinogenic Risks to Humans (2013) Non-ionizing radiation. (Part 2: radiofrequency electromagnetic fields), vol 102. IARC, Lyon
SCENIHR (2015) Scientific Committee on emerging and newly identified health risks: potential health effects of eExposure to Electromagnetic Fields (EMF). http://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_041.pdf. Accessed 15 Aug 2015
Interphone Study Group (2010) Brain tumour risk in relation to mobile telephone use: results of the INTERPHONE international case-control study. Int J Epidemiol 39(3):675–694
Feychting M, Schüz J (2017) Chap. 15: electromagnetic fields. In: Thun M, Linet MS, Cerhan JR, Haiman CA, Schottenfeld D (eds) Cancer epidemiology and prevention, 4th edn. Oxford Scholarship, Oxford. https://doi.org/10.1093/oso/9780190238667.001.0001
Hardell L, Carlberg M (2013) Use of mobile and cordless phones and survival of patients with glioma. Neuroepidemiology 40(2):101–108
Salford LG, Brun A, Persson BRR, Eberhardt J (1993) Experimental studies of brain tumour development during exposure to continuous and pulsed 915 MHz radiofrequency radiation. Bioelectrochem Bioenerg 30:313–318
Higashikubo R, Culbreth VO, Spitz DR, LaRegina MC, Pickard WF, Straube WL et al (1999) Radiofrequency electromagnetic fields have no effect on the in vivo proliferation of the 9L brain tumor. Radiat Res 152(6):665–671
Simko M, Remondini D, Zeni O, Scarfi MR (2016) Quality matters: systematic analysis of endpoints related to “cellular life” in vitro data of radiofrequency electromagnetic field exposure. Int J Environ Res Public Health 13(7):701–717
Kobayashi T, Masumoto J, Tada T, Nomiyama T, Hongo K, Nakayama J (2007) Prognostic significance of the immunohistochemical staining of cleaved caspase-3, an activated form of caspase-3, in gliomas. Clin Cancer Res 13(13):3868–3874
Liu YX, Tai JL, Li GQ, Zhang ZW, Xue JH, Liu HS et al (2012) Exposure to 1950-MHz TD-SCDMA electromagnetic fields affects the apoptosis of astrocytes via caspase-3-dependent pathway. PLoS ONE 7(8):e42332
Stagg RB, Thomas WJ, Jones RA, Adey WR (1997) DNA synthesis and cell proliferation in C6 glioma and primary glial cells exposed to a 836.55 MHz modulated radiofrequency field. Bioelectromagnetics 18(3):230–236
Dasdag S, Akdag MZ, Ulukaya E, Uzunlar AK, Ocak AR (2009) Effect of mobile phone exposure on apoptotic glial cells and status of oxidative stress in rat brain. Electromagn Biol Med 28(4):342–354
Ahmed S, Rashed H, Hegazy A, Mohamed AM, Elmesallamy W (2016) Prognostic value of ALDH1, EZH2 and Ki-67 in astrocytic gliomas. Turk Patol Derg 32(2):70–81
Capri M, Scarcella E, Fumelli C, Bianchi E, Salvioli S, Mesirca P et al (2004) In vitro exposure of human lymphocytes to 900 MHz CW and GSM modulated radiofrequency: studies of proliferation, apoptosis and mitochondrial membrane potential. Radiat Res 162(2):211–218
Zeni O, Romano M, Perrotta A, Lioi MB, Barbieri R, d’Ambrosio G et al (2005) Evaluation of genotoxic effects in human peripheral blood leukocytes following an acute in vitro exposure to 900 MHz radiofrequency fields. Bioelectromagnetics 26(4):258–265
Eghlidospour M, Ghanbari A, Mortazavi SMJ, Azari H (2017) Effects of radiofrequency exposure emitted from a GSM mobile phone on proliferation, differentiation, and apoptosis of neural stem cells. Anat Cell Biol 50(2):115–123
Sanchez S, Masuda H, Billaudel B, Haro E, Anane R, Leveque P et al (2006) Effect of GSM-900 and – 1800 signals on the skin of hairless rats. II: 12-week chronic exposures. Int J Radiat Biol 82(9):675–680
Sanchez S, Milochau A, Ruffie G, Poulletier de Gannes F, Lagroye I, Haro E et al (2006) Human skin cell stress response to GSM-900 mobile phone signals. In vitro study on isolated primary cells and reconstructed epidermis. FEBS J 273(24):5491–5507
Das S, Marsden PA (2013) Angiogenesis in glioblastoma. N Engl J Med 369(16):1561–1563
Rao A, Manyam G, Rao G, Jain R (2016) Integrative analysis of mRNA, microRNA, and protein correlates of relative cerebral blood volume values in GBM reveals the role for modulators of angiogenesis and tumor proliferation. Cancer Inform 15:29–33
Rong Y, Durden DL, Van Meir EG, Brat DJ (2006) ‘Pseudopalisading’ necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J Neuropathol Exp Neurol 65(6):529–539
Tastekin E, Caloglu VY, Puyan FO, Tokuc B, Caloglu M, Yalta TD et al (2016) Prognostic value of angiogenesis and survivin expression in patients with glioblastoma. Turk Neurosurg 26(4):484–490
Tepper OM, Callaghan MJ, Chang EI, Galiano RD, Bhatt KA, Baharestani S et al (2004) Electromagnetic fields increase in vitro and in vivo angiogenesis through endothelial release of FGF-2. FASEB J 18(11):1231–1233
Barthelemy A, Mouchard A, Bouji M, Blazy K, Puigsegur R, Villegier AS (2016) Glial markers and emotional memory in rats following acute cerebral radiofrequency exposures. Environ Sci Pollut Res Int 23(24):25343–25355
Lu Y, He M, Zhang Y, Xu S, Zhang L, He Y et al (2014) Differential pro-inflammatory responses of astrocytes and microglia involve STAT3 activation in response to 1800 MHz radiofrequency fields. PLoS ONE 9(9):e108318
Sowers JL, Johnson KM, Conrad C, Patterson JT, Sowers LC (2014) The role of inflammation in brain cancer. Adv Exp Med Biol 816:75–105
Maskey D, Pradhan J, Aryal B, Lee CM, Choi IY, Park KS et al (2010) Chronic 835-MHz radiofrequency exposure to mice hippocampus alters the distribution of calbindin and GFAP immunoreactivity. Brain Res 1346:237–246
Jacobs VL, Valdes PA, Hickey WF, De Leo JA (2011) Current review of in vivo GBM rodent models: emphasis on the CNS-1 tumour model. ASN Neuro 3(3):e00063
Leveque PDC, Veyret B, Wiart J (2004) Dosimetric analysis of a 900-MHz rat head exposure system. IEEE Trans Microw Theory Tech 52(8):2076–2083
Grobben B, De Deyn PP, Slegers H (2002) Rat C6 glioma as experimental model system for the study of glioblastoma growth and invasion. Cell Tissue Res 310(3):257–270
Paxinos G, Watsen C (2006) The rat brain in stereotaxic coordinates. Hard cover edition. Academic Press, San Diego
Olsson A, Bouaoun L, Auvinen A, Feychting M, Johansen C, Mathiesen T, Melin B, Lahkola A, Larjavaara S, Villégier AS, Deltour I, Schüz J (2018) Survival of glioma patients in relation to mobile phone use in in Denmark, Finland and Sweden. https://doi.org/10.1007/s11060-018-03019-5
Burger PC (1987) The anatomy of astrocytomas. Mayo Clin Proc 62(6):527–529
Bouji M, Lecomte A, Gamez C, Blazy K, Villegier AS (2016) Neurobiological effects of repeated radiofrequency exposures in male senescent rats. Biogerontology 17(5–6):841–857
Petitdant N, Lecomte A, Robidel F, Gamez C, Blazy K, Villegier AS (2016) Cerebral radiofrequency exposures during adolescence: impact on astrocytes and brain functions in healthy and pathologic rat models. Bioelectromagnetics 37(5):338–350
Acknowledgements
This work was financed by the French National Research Program for Environmental and Occupational Health of ANSES [2014/2 RF/002] and French Ministry of Ecology [Program 190].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ouadah, N.S., Lecomte, A., Robidel, F. et al. Possible effects of radiofrequency electromagnetic fields on in vivo C6 brain tumors in Wistar rats. J Neurooncol 140, 539–546 (2018). https://doi.org/10.1007/s11060-018-03012-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-03012-y