Abstract
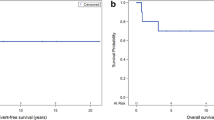
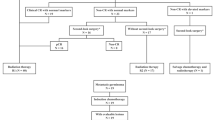
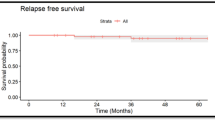
Craniospinal irradiation is standard radiotherapy (RT) for localized intracranial nongerminoma germ cell tumors (NGGCT). Given its toxicity, there is interest in using smaller fields. We examined outcomes of NGGCT patients receiving reduced-volume RT at a single institution. Records of 16 patients who received reduced-volume RT as part of definitive treatment between 1996 and 2016 were reviewed. Median age at presentation was 10.8 years (range 4.6–41.0 years). Ten patients had pineal tumors and 6 had suprasellar tumors. All received chemotherapy and 9 patients received second-look surgery thereafter. RT volume was tumor-only to a median of 54 Gy (range 50.4–54 Gy) in 3 patients and whole-ventricle irradiation to a median of 30.6 Gy (range 30.6–36 Gy) with a boost to 54 Gy in 13 patients. Median follow-up was 4.1 years (range 1.9–19.3 years). Three patients recurred locally at a median 9.9 months (range 9.6–10.6 months) after diagnosis, and one of these developed leptomeningeal relapse after 30 months. One patient expired from disease 2.6 years post-diagnosis and another due to stroke 19.3 years post-diagnosis. Fourteen patients are alive with no evidence of disease. Kaplan–Meier estimates of the 4-year overall survival and failure-free survival are 92% (95% confidence interval [CI], 57–99%) and 81% (95% CI 53–94%), respectively. Excellent disease control was observed in these patients with no initial relapses outside of these RT fields. The results of ACNS1123 may better delineate patterns of failure and identify subgroups likely to benefit from this approach.

Similar content being viewed by others
References
Jennings MT, Gelman R, Hochberg F (1985) Intracranial germ-cell tumors: natural history and pathogenesis. J Neurosurg 63(2):155–167. doi:10.3171/jns.1985.63.2.0155
Kamoshima Y, Sawamura Y (2010) Update on current standard treatments in central nervous system germ cell tumors. Curr Opin Neurol 23(6):571–575. doi:10.1097/WCO.0b013e32833ff522
Echevarria ME, Fangusaro J, Goldman S (2008) Pediatric central nervous system germ cell tumors: a review. Oncologist 13(6):690–699. doi:10.1634/theoncologist.2008-0037
Schild SE, Haddock MG, Scheithauer BW, Marks LB, Norman MG, Burger PC, Wong WW, Lyons MK, Schomberg PJ (1996) Nongerminomatous germ cell tumors of the brain. Int J Radiat Oncol Biol Phys 36(3):557–563
Matsutani M, Sano K, Takakura K, Fujimaki T, Nakamura O, Funata N, Seto T (1997) Primary intracranial germ cell tumors: a clinical analysis of 153 histologically verified cases. J Neurosurg 86(3):446–455. doi:10.3171/jns.1997.86.3.0446
Drummond KJ, Rosenfeld JV (1999) Pineal region tumours in childhood. A 30-year experience. Child’s Nerv Syst 15(2–3):119–126; (discussion 127)
Jaing TH, Wang HS, Hung IJ, Tseng CK, Yang CP, Hung PC, Lui TN (2002) Intracranial germ cell tumors: a retrospective study of 44 children. Pediatr Neurol 26(5):369–373
Packer RJ, Cohen BH, Cooney K (2000) Intracranial germ cell tumors. Oncologist 5(4):312–320
Fossati P, Ricardi U, Orecchia R (2009) Pediatric medulloblastoma: toxicity of current treatment and potential role of protontherapy. Cancer Treat Rev 35(1):79–96. doi:10.1016/j.ctrv.2008.09.002
Calaminus G, Bamberg M, Baranzelli MC, Benoit Y, di Montezemolo LC, Fossati-Bellani F, Jurgens H, Kuhl HJ, Lenard HG, Curto ML et al (1994) Intracranial germ cell tumors: a comprehensive update of the European data. Neuropediatrics 25(1):26–32. doi:10.1055/s-2008-1071577
Buckner JC, Peethambaram PP, Smithson WA, Groover RV, Schomberg PJ, Kimmel DW, Raffel C, O’Fallon JR, Neglia J, Shaw EG (1999) Phase II trial of primary chemotherapy followed by reduced-dose radiation for CNS germ cell tumors. J Clin Oncol 17(3):933–940
Kellie SJ, Boyce H, Dunkel IJ, Diez B, Rosenblum M, Brualdi L, Finlay JL (2004) Primary chemotherapy for intracranial nongerminomatous germ cell tumors: results of the second international CNS germ cell study group protocol. J Clin Oncol 22(5):846–853. doi:10.1200/JCO.2004.07.006
Goldman S, Bouffet E, Fisher PG, Allen JC, Robertson PL, Chuba PJ, Donahue B, Kretschmar CS, Zhou T, Buxton AB, Pollack IF (2015) Phase II trial assessing the ability of neoadjuvant chemotherapy with or without second-look surgery to eliminate measurable disease for nongerminomatous germ cell tumors: A Children’s Oncology Group Study. J Clin Oncol 33(22):2464–2471. doi:10.1200/JCO.2014.59.5132
Chemotherapy followed by radiation therapy in treating younger patients with newly diagnosed localized central nervous system germ cell tumors. https://ClinicalTrials.gov/show/NCT01602666
Merchant TE, Hodgson D, Laack NN, Wolden S, Indelicato DJ, Kalapurakal JA (2013) Children’s Oncology Group’s 2013 blueprint for research: radiation oncology. Pediatr Blood Cancer 60(6):1037–1043. doi:10.1002/pbc.24425
Calaminus G, Frappaz D, Kortmann RD, Alapetite C, Garre ML, Ricardi U, Saran FH, Nicholson J (2012) GC-11. Risk adapted irradiation is feasible in intracranial non-germinomatous germ cell tumours (NGGCT): final results of SIOP CNS GCT 96. Neuro-oncology 14(Suppl 1):i49–i55. doi:10.1093/neuonc/nos101
Aizer AA, Sethi RV, Hedley-Whyte ET, Ebb D, Tarbell NJ, Yock TI, MacDonald SM (2013) Bifocal intracranial tumors of nongerminomatous germ cell etiology: diagnostic and therapeutic implications. Neuro-oncology 15(7):955–960. doi:10.1093/neuonc/not050
Robertson PL, DaRosso RC, Allen JC (1997) Improved prognosis of intracranial non-germinoma germ cell tumors with multimodality therapy. J Neurooncol 32(1):71–80
Calaminus G, Bamberg M, Harms D, Jürgens H, Kortmann RD, Sörensen N, Wiestler OD, Göbel U (2005) AFP/β-HCG secreting CNS germ cell tumors: long-term outcome with respect to initial symptoms and primary tumor resection. Results of the cooperative trial MAKEI 89. Neuropediatrics 36(02):71–77. doi:10.1055/s-2005-837582
Patte C, Frappaz D, Raquin MA, Bouffet E, Kalifa C, Baranzelli MC (2002) Treatment of primary intracranial germ cell tumours with carboplatin-based chemotherapy and focal irradiation. In: Harnden P, Joffe JK, Jones WG (eds) Germ cell tumours V: the proceedings of the Fifth Germ Cell Tumour Conference Devonshire Hall, University of Leeds, 13th–15th September, 2001. Springer, London, pp 131–131. doi:10.1007/978-1-4471-3281-3_29
Robertson PL, Jakacki R, Hukin J, Siffert J, Allen JC (2014) Multimodality therapy for CNS mixed malignant germ cell tumors (MMGCT): results of a phase II multi-institutional study. J Neurooncol 118(1):93–100. doi:10.1007/s11060-013-1306-0
Hoffman HJ, Otsubo H, Hendrick EB, Humphreys RP, Drake JM, Becker LE, Greenberg M, Jenkin D (1991) Intracranial germ-cell tumors in children. J Neurosurg 74(4):545–551. doi:10.3171/jns.1991.74.4.0545
Dearnaley DP, A’Hern RP, Whittaker S, Bloom HJ (1990) Pineal and CNS germ cell tumors: Royal Marsden Hospital experience 1962–1987. Int J Radiat Oncol Biol Phys 18(4):773–781
Chen MJ, Santos Ada S, Sakuraba RK, Lopes CP, Goncalves VD, Weltman E, Ferrigno R, Cruz JC (2010) Intensity-modulated and 3D-conformal radiotherapy for whole-ventricular irradiation as compared with conventional whole-brain irradiation in the management of localized central nervous system germ cell tumors. Int J Radiat Oncol Biol Phys 76(2):608–614. doi:10.1016/j.ijrobp.2009.06.028
Yang JC, Terezakis SA, Dunkel IJ, Gilheeney SW, Wolden SL (2016) Intensity-modulated radiation therapy with dose painting: a brain-sparing technique for intracranial germ cell tumors. Pediatr Blood Cancer 63(4):646–651. doi:10.1002/pbc.25867
Kim JY, Park J (2015) Understanding the treatment strategies of intracranial germ cell tumors: focusing on radiotherapy. J Korean Neurosurg Soc 57(5):315–322. doi:10.3340/jkns.2015.57.5.315
Acknowledgements
Funding was provided by the National Institutes of Health/National Cancer Institute Cancer Center Support Grant (P30 CA008748). This work is also supported by a gift from Jack and Susan Rudin
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ira Dunkel reports personal fees from Bayer Health Care Pharmaceuticals, Bristol-Myers Squibb, Ipsen, Eisai, and Pfizer, and grants from Genentech and Parexel (GSK, Novartis), all outside the submitted work. Mark Souweidane reports personal fees from Aesculap outside the submitted work. All other authors declare no conflicts.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
De, B., Cahlon, O., Dunkel, I.J. et al. Reduced-volume radiotherapy for patients with localized intracranial nongerminoma germ cell tumors. J Neurooncol 134, 349–356 (2017). https://doi.org/10.1007/s11060-017-2532-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2532-7