Abstract
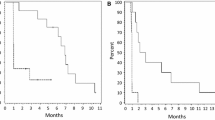
Angiogenesis, a hallmark of glioblastoma, can potentially be targeted by inhibiting the VEGF pathway using bevacizumab, a humanized monoclonal antibody against VEGF-A. This study was designed to determine the efficacy and safety of these regimens in the cooperative group setting. Eligibility included age ≥18, recurrent or progressive GBM after standard chemoradiation. Treatment was intravenous bevacizumab 10 mg/kg and either irinotecan (CPT) 125 mg/m2 every 2 weeks or temozolomide (TMZ) 75–100 mg/m2 day 1–21 of 28 day cycle. Accrual goal was 57 eligible patients per arm. Primary endpoint was 6 month progression-free survival (6-m PFS); a predetermined rate of ≥35 % to declare efficacy. 60 eligible patients were enrolled on TMZ arm and 57 patients on CPT arm. Median age was 56, median KPS was 80. For TMZ arm, the 6-m-PFS rate was 39 % (23/59); for the CPT arm, the 6-m-PFS rate was 38.6 % (22/57). Objective responses: TMZ arm had 2 (3 %) CR, 9 (16 %) PR; CPT arm had 2 (4 %) CR, 13 (24 %) PR. Overall there was moderate toxicity: TMZ arm with 33 (55 %) grade 3, 11 (18 %) grade 4, and 1 (2 %) grade 5 (fatal) toxicities; CPT arm had 22 (39 %) grade 3, 7 (12 %) grade 4, and 3 (5 %) grade 5 toxicities. The 6-m-PFS surpassed the predetermined efficacy threshold for both arms, corroborating the efficacy of bevacizumab and CPT and confirming activity for bevacizumab and protracted TMZ for recurrent/progressive GBM, even after prior temozolomide exposure. Toxicities were within anticipated frequencies with a moderately high rate of venous thrombosis, moderate hypertension and one intracranial hemorrhage.



Similar content being viewed by others
References
Wong ET, Hess KR, Gleason MJ et al (1999) Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 17:2572
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359:492–507
Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT (2007) Angiogenesis in brain tumours. Nat Rev Neurosci 8:610–622
Visted T, Lund-Johansen M (2003) Progress and challenges for cell encapsulation in brain tumour therapy. Expert Opin Biol Ther 3:551–561
Jain RK (2005) Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307:58–62
Kerbel RS, Kamen BA (2004) The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer 4:423–436
Folkins C, Man S, Xu P, Shaked Y, Hicklin DJ, Kerbel R (2007) Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res 67:3560–3564
Gilbertson RJ, Rich JN (2007) Making a tumour’s bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer 7:733–736
Kreisl TN, Kim L, Moore K et al (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27:740–745
Friedman HS, Prados MD, Wen PY et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740
Armstrong TS, Wen PY, Gilbert MR, Schiff D (2012) Management of treatment-associated toxicites of anti-angiogenic therapy in patients with brain tumors. Neuro-oncology 14:1203–1214
Cohen MH, Shen YL, Keegan P, Pazdur R (2009) FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist 14:1131–1138
Tol J, Punt CJ (2010) Monoclonal antibodies in the treatment of metastatic colorectal cancer: a review. Clin Ther 32:437–453
Reardon DA, Turner S, Peters KB et al (2011) A review of VEGF/VEGFR-targeted therapeutics for recurrent glioblastoma. J Natl Compr Canc Netw 9:414–427
Gilbert MR, Dignam JJ, Armstrong TS et al (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370:699–708
Chinot OL, Wick W, Mason W et al (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370:709–722
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Zelen M (1974) The randomization and stratification of patients to clinical trials. J Chronic Dis 27:365–375
Fleming TR (1982) One-sample multiple testing procedure for phase II clinical trials. Biometrics 38:143–151
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Vredenburgh JJ, Desjardins A, Herndon JE 2nd et al (2007) Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res 13:1253–1259
Yung WK, Albright RE, Olson J et al (2000) A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer 83:588–593
Taal W, Oosterkamp HM, Walenkamp AM et al (2014) Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol 15:943–953
Wick W, Brandes AA, Gorlia T et al (2015) Phase III trial exploring the combination of bevacizumab and lomustine in patients with first recurrence of a glioblastoma: the EORTC 26101 trial. Neuro Oncol 17(suppl 5):v1
Acknowledgments
We would like to thank the NRG Oncology RTOG staff including Minhee Won, Kathryn Okrent, Sandrine Geinoz, Barbara Kaiser, and Denise Manfredi for their outstanding work on this clinical trial.
Funding
This project was supported by grants U10CA21661, U10CA180868, U10CA180822, U10CA37422, and U24CA180803 from the National Cancer Institute (NCI).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Gilbert reports personal fees and non-financial support from Merck, personal fees from Genentech Roche, personal fees from Abbvie, personal fees from Wellcome Trust, and personal fees from Foundation Medicine, outside the submitted work. Dr. Sorensen reports employment by Siemens Healthcare, outside the submitted work. Dr. Mikkelsen has a consulting or advisory role with Roche Genentech and has received honoraria, travel and research funding from Roche Genentech, outside the submitted work. Dr. Penas-Prado has received research funding from Bayer, Genentech, Glaxo, and Novartis, outside the submitted work. Dr. Mehta has a leadership role with Pharmacyclics, stock or ownership interest in Pharmacyclics, consulting or advisory roles with Cavion, Elekta, Novartis and Novocure, and has received research funding from Novocure and Novellos, outside the submitted work.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Gilbert, M.R., Pugh, S.L., Aldape, K. et al. NRG oncology RTOG 0625: a randomized phase II trial of bevacizumab with either irinotecan or dose-dense temozolomide in recurrent glioblastoma. J Neurooncol 131, 193–199 (2017). https://doi.org/10.1007/s11060-016-2288-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2288-5