Abstract
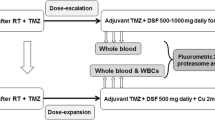
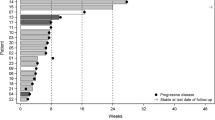
Disulfiram, a generic alcohol aversion drug, has promising preclinical activity against glioblastoma (GBM). This phase I study aims to evaluate its safety, maximum tolerated dose (MTD), pharmacodynamic effect, and preliminary efficacy when combined with adjuvant temozolomide in GBM patients after standard chemoradiotherapy. Patients received disulfiram 500–1000 mg once daily, in combination with 150–200 mg/m2 temozolomide. A modified 3 + 3 dose-escalation design was used to determine the MTD. The pharmacodynamic effect of proteasome inhibition was assessed using fluorometric 20S proteasome assay on peripheral blood cells. The MTD was determined based on the dose-limiting toxicities (DLTs) within the first month of therapy. Twelve patients were enrolled to two dose levels: 500 and 1000 mg. Two DLTs of grade 3 delirium occurred after 15 days of administration at 1000 mg per day. Other possible grade 2–3 DSF-related toxicities included fatigue, ataxia, dizziness, and peripheral neuropathy. The toxicities were self-limiting or resolved after discontinuing DSF. The MTD was determined to be 500 mg per day. Limited proteasome inhibition was observed at week 4 and showed an increased trend with escalated disulfiram. Median progression-free survival with 500 mg of DSF was 5.4 months from the start of disulfiram and 8.1 months from the start of chemoradiotherapy. Disulfiram can be safely combined with temozolomide but can cause reversible neurological toxicities. The MTD of disulfiram with adjuvant temozolomide appears to produce limited proteasome inhibition on peripheral blood cells.


Similar content being viewed by others
References
Johnson DR, O’Neill BP (2012) Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol 107:359–364. doi:10.1007/s11060-011-0749-4
CBTRUS Statistical Report (2011): primary brain and central nervous system tumors diagnosed in the United States in 2004–2007. www.cbtrus.org
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. doi:10.1056/NEJMoa043330
DiMasi JA, Grabowski HG (2007) Economics of new oncology drug development. J Clin Oncol 25:209–216. doi:10.1200/JCO.2006.09.0803
Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP, Vikas P (2014) The repurposing drugs in oncology (ReDO) project. Ecancermedicalscience 8:442. doi:10.3332/ecancer.2014.442
Sharma A, Jacob A, Tandon M, Kumar D (2010) Orphan drug: development trends and strategies. J Pharm Bioallied Sci 2:290–299. doi:10.4103/0975-7406.72128
Kast RE, Boockvar JA, Bruning A, Cappello F, Chang WW, Cvek B, Dou QP, Duenas-Gonzalez A, Efferth T, Focosi D, Ghaffari SH, Karpel-Massler G, Ketola K, Khoshnevisan A, Keizman D, Magne N, Marosi C, McDonald K, Munoz M, Paranjpe A, Pourgholami MH, Sardi I, Sella A, Srivenugopal KS, Tuccori M, Wang W, Wirtz CR, Halatsch ME (2013) A conceptually new treatment approach for relapsed glioblastoma: coordinated undermining of survival paths with nine repurposed drugs (CUSP9) by the International Initiative for Accelerated Improvement of Glioblastoma Care. Oncotarget 4:502–530
Triscott J, Rose Pambid M, Dunn SE (2015) Concise review: bullseye: targeting cancer stem cells to improve the treatment of gliomas by repurposing disulfiram. Stem Cells 33:1042–1046. doi:10.1002/stem.1956
Faiman MD, Dodd DE, Hanzlik RE (1978) Distribution of S35 disulfiram and metabolites in mice, and metabolism of S35 disulfiram in the dog. Res Commun Chem Pathol Pharmacol 21:543–567
Suh JJ, Pettinati HM, Kampman KM, O’Brien CP (2006) The status of disulfiram: a half of a century later. J Clin Psychopharmacol 26:290–302. doi:10.1097/01.jcp.0000222512.25649.08
Hothi P, Martins TJ, Chen L, Deleyrolle L, Yoon JG, Reynolds B, Foltz G (2012) High-throughput chemical screens identify disulfiram as an inhibitor of human glioblastoma stem cells. Oncotarget 3:1124–1136
Liu P, Brown S, Goktug T, Channathodiyil P, Kannappan V, Hugnot JP, Guichet PO, Bian X, Armesilla AL, Darling JL, Wang W (2012) Cytotoxic effect of disulfiram/copper on human glioblastoma cell lines and ALDH-positive cancer-stem-like cells. Br J Cancer 107:1488–1497. doi:10.1038/bjc.2012.442
Triscott J, Lee C, Hu K, Fotovati A, Berns R, Pambid M, Luk M, Kast RE, Kong E, Toyota E, Yip S, Toyota B, Dunn SE (2012) Disulfiram, a drug widely used to control alcoholism, suppresses the self-renewal of glioblastoma and over-rides resistance to temozolomide. Oncotarget 3:1112–1123
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444:756–760. doi:10.1038/nature05236
Chen D, Cui QC, Yang H, Dou QP (2006) Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res 66:10425–10433. doi:10.1158/0008-5472.CAN-06-2126
Cvek B, Milacic V, Taraba J, Dou QP (2008) Ni(II), Cu(II), and Zn(II) diethyldithiocarbamate complexes show various activities against the proteasome in breast cancer cells. J Med Chem 51:6256–6258. doi:10.1021/jm8007807
Lun X, Wells JC, Hao X, Zhang J, Grinshtein N, Kaplan D, Luchman A, Weiss S, Cairncross JG, Senger DS, Robbins S (2013) Disulfiram when combined with copper is an effective adjuvant therapy with TMZ for treatment of human gliomblastoma. Neuro-oncology 15(Suppl 3):iii52 [Abstract: ET-064]
Paranjpe A, Zhang R, Ali-Osman F, Bobustuc GC, Srivenugopal KS (2014) Disulfiram is a direct and potent inhibitor of human O6-methylguanine-DNA methyltransferase (MGMT) in brain tumor cells and mouse brain and markedly increases the alkylating DNA damage. Carcinogenesis 35:692–702. doi:10.1093/carcin/bgt366
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972. doi:10.1200/JCO.2009.26.3541
Lightcap ES, McCormack TA, Pien CS, Chau V, Adams J, Elliott PJ (2000) Proteasome inhibition measurements: clinical application. Clin Chem 46:673–683
Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M, Rekhtman G, Masliak Z, Robak T, Shubina A, Arnulf B, Kropff M, Cavet J, Esseltine DL, Feng H, Girgis S, van de Velde H, Deraedt W, Harousseau JL (2011) Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol 12:431–440. doi:10.1016/S1470-2045(11)70081-X
Orlowski RZ, Stinchcombe TE, Mitchell BS, Shea TC, Baldwin AS, Stahl S, Adams J, Esseltine DL, Elliott PJ, Pien CS, Guerciolini R, Anderson JK, Depcik-Smith ND, Bhagat R, Lehman MJ, Novick SC, O’Connor OA, Soignet SL (2002) Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol 20:4420–4427
Schweizer MT, Lin J, Blackford A, Bardia A, King S, Armstrong AJ, Rudek MA, Yegnasubramanian S, Carducci MA (2013) Pharmacodynamic study of disulfiram in men with non-metastatic recurrent prostate cancer. Prostate Cancer Prostatic Dis 16:357–361. doi:10.1038/pcan.2013.28
Stewart DJ, Verma S, Maroun JA (1987) Phase I study of the combination of disulfiram with cisplatin. Am J Clin Oncol 10:517–519
Nilsson GE, Tottmar O, Wahlstrom G (1987) Effects of aldehyde dehydrogenase inhibitors on hexobarbital sensitivity and neuroamine metabolism in rat brain. Brain Res 409:265–274
Major LF, Lerner P, Ballenger JC, Brown GL, Goodwin FK, Lovenberg W (1979) Dopamine-beta-hydroxylase in the cerebrospinal fluid: relationship to disulfiram-induced psychosis. Biol Psychiatry 14:337–344
Faiman MD, Jensen JC, Lacoursiere RB (1984) Elimination kinetics of disulfiram in alcoholics after single and repeated doses. Clin Pharmacol Ther 36:520–526
Choi SA, Choi JW, Wang KC, Phi JH, Lee JY, Park KD, Eum D, Park SH, Kim IH, Kim SK (2015) Disulfiram modulates stemness and metabolism of brain tumor initiating cells in atypical teratoid/rhabdoid tumors. Neuro-oncology 17:810–821. doi:10.1093/neuonc/nou305
Kim SK, Kim H, Lee DH, Kim TS, Kim T, Chung C, Koh GY, Kim H, Lim DS (2013) Reversing the intractable nature of pancreatic cancer by selectively targeting ALDH-high, therapy-resistant cancer cells. PLoS One 8:e78130. doi:10.1371/journal.pone.0078130
Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, Armstrong TS, Wefel JS, Won M, Blumenthal DT, Mahajan A, Schultz CJ, Erridge S, Baumert B, Hopkins KI, Tzuk-Shina T, Brown PD, Chakravarti A, Curran WJ Jr, Mehta MP (2013) Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol 31:4085–4091. doi:10.1200/JCO.2013.49.6968
Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, Jeraj R, Brown PD, Jaeckle KA, Schiff D, Stieber VW, Brachman DG, Werner-Wasik M, Tremont-Lukats IW, Sulman EP, Aldape KD, Curran WJ Jr, Mehta MP (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370:699–708. doi:10.1056/NEJMoa1308573
Acknowledgments
The authors would like to thank Dr. Eric Lightcap for his helpful discussions on proteasome activity assay. We would like to thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, MO., for the use of the Clinical Trials Office, which provided protocol development, regulatory, data management, and study coordination services. We would like to specifically acknowledge Stephanie Myles, Bethany Rensink, Sarah Marchetti, and Maria Miller of the Siteman Cancer Center for their work on this trial. The Siteman Cancer Center is supported in part by a NCI Cancer Center Support Grant #P30 CA091842. We would also like to acknowledge our courageous patients and their families for their inspiration.
Funding
Institutional fund from the Department of Radiation Oncology, Washington University School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
We have no conflict of interests to disclose for this work.
Additional information
Jian L. Campian and Amit D. Gujar contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, J., Campian, J.L., Gujar, A.D. et al. A phase I study to repurpose disulfiram in combination with temozolomide to treat newly diagnosed glioblastoma after chemoradiotherapy. J Neurooncol 128, 259–266 (2016). https://doi.org/10.1007/s11060-016-2104-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2104-2