Abstract
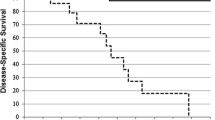
Because of the rarity of intracranial hemangiopericytomas (HPCs), the role of postoperative radiation therapy (PORT) in the management of HPC remains unclear. This study therefore analyzed the effects of PORT on patterns of failure and survival improvement in patients with HPC. Fifty-two patients surgically treated for intracranial HPC at our institution between 1992 and 2013 were retrospectively analyzed. Patterns of failure were subdivided into local recurrence, regional metastasis, and distant metastasis. Multivariate Cox proportional hazards models were used to assess factors prognostic of treatment failure and survival, and a time-dependent Cox proportional hazards models were used to investigate the correlations between patterns of failure and death. Of the 52 patients, 45 (87 %) underwent gross total resection, and 39 (75 %) received PORT. PORT significantly lengthened local control (LC) and overall survival (OS), by 14 and 13 months, respectively, independent of the extent of resection. Patients who did and did not receive PORT had 5 year LC rates of 97 and 44 %, respectively (HR .05, P = .002); and 10 year OS rates of 83 and 25 %, respectively (hazard ratio (HR) .20, P = .008). PORT, however, did not show preventive effects on regional and distant metastases. The main patterns of failure were local recurrence in patients who did not receive PORT and distant metastasis in those who received PORT. Regional metastasis was a main immediate cause of death (P < .001), and tended to occur more frequently and earlier in patients not receiving PORT.


Similar content being viewed by others
References
Chan RC, Thompson GB (1984) Morbidity, mortality, and quality of life following surgery for intracranial meningiomas. A retrospective study in 257 cases. J Neurosurg 60:52–60. doi:10.3171/jns.1984.60.1.0052
Guthrie BL, Ebersold MJ, Scheithauer BW, Shaw EG (1989) Meningeal hemangiopericytoma: histopathological features, treatment, and long-term follow-up of 44 cases. Neurosurgery 25:514–522
Jaaskelainen J, Servo A, Haltia M, Wahlstrom T, Valtonen S (1985) Intracranial hemangiopericytoma: radiology, surgery, radiotherapy, and outcome in 21 patients. Surg Neurol 23:227–236
Kim JH, Jung HW, Kim YS, Kim CJ, Hwang SK, Paek SH, Kim DG, Kwun BD (2003) Meningeal hemangiopericytomas: long-term outcome and biological behavior. Surg Neurol 59:47–53 discussion 53-44
Sonabend AM, Zacharia BE, Goldstein H, Bruce SS, Hershman D, Neugut AI, Bruce JN (2014) The role for adjuvant radiotherapy in the treatment of hemangiopericytoma: a surveillance, epidemiology, and end results analysis. J Neurosurg 120:300–308. doi:10.3171/2013.10.JNS13113
Vuorinen V, Sallinen P, Haapasalo H, Visakorpi T, Kallio M, Jaaskelainen J (1996) Outcome of 31 intracranial haemangiopericytomas: poor predictive value of cell proliferation indices. Acta Neurochir 138:1399–1408
Staples JJ, Robinson RA, Wen BC, Hussey DH (1990) Hemangiopericytoma—the role of radiotherapy. Int J Radiat Oncol Biol Phys 19:445–451. doi:10.1089/ten.2005.11.985
Schiariti M, Goetz P, El-Maghraby H, Tailor J, Kitchen N (2011) Hemangiopericytoma: long-term outcome revisited. J Neurosurg 114:747–755. doi:10.3171/2010.6.JNS091660
Ghia AJ, Allen PK, Mahajan A, Penas-Prado M, McCutcheon IE, Brown PD (2013) Intracranial hemangiopericytoma and the role of radiation therapy: a population based analysis. Neurosurgery 72:203–209. doi:10.1227/NEU.0b013e31827b9e68
Stessin AM, Sison C, Nieto J, Raifu M, Li B (2013) The role of postoperative radiation therapy in the treatment of meningeal hemangiopericytoma-experience from the SEER database. Int J Radiat Oncol Biol Phys 85:784–790. doi:10.1016/j.ijrobp.2012.05.042
Ghia AJ, Chang EL, Allen PK, Mahajan A, Penas-Prado M, McCutcheon IE, Brown PD (2013) Intracranial hemangiopericytoma: patterns of failure and the role of radiation therapy. Neurosurgery 73:624–630. doi:10.1227/NEU.0000000000000064 discussion 630-621
Soyuer S, Chang EL, Selek U, McCutcheon IE, Maor MH (2004) Intracranial meningeal hemangiopericytoma: the role of radiotherapy: report of 29 cases and review of the literature. Cancer 100:1491–1497. doi:10.1002/cncr.20109
Giannini C, Rushing E, Hainfellner J (2007) Haemangiopericytoma. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds) WHO classification of tumours of the central nervous system. IARC Press, Lyon
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109. doi:10.1007/s00401-007-0243-4
National cancer institute (2010) Common terminology criteria for adverse events (CTCAE) v.4 data files. http://evs.nci.nih.gov/ftp1/CTCAE/About.html. Accessed 8 December 2015
Melone AG, D’Elia A, Santoro F, Salvati M, Delfini R, Cantore G, Santoro A (2014) Intracranial hemangiopericytoma—our experience in 30 years: a series of 43 cases and review of the literature. World Neurosurg 81:556–562. doi:10.1016/j.wneu.2013.11.009
Rutkowski MJ, Jian BJ, Bloch O, Chen C, Sughrue ME, Tihan T, Barani IJ, Berger MS, McDermott MW, Parsa AT (2012) Intracranial hemangiopericytoma: clinical experience and treatment considerations in a modern series of 40 adult patients. Cancer 118:1628–1636. doi:10.1002/cncr.26411
Rutkowski MJ, Sughrue ME, Kane AJ, Aranda D, Mills SA, Barani IJ, Parsa AT (2010) Predictors of mortality following treatment of intracranial hemangiopericytoma. J Neurosurg 113:333–339. doi:10.3171/2010.3.JNS091882
Olson C, Yen CP, Schlesinger D, Sheehan J (2010) Radiosurgery for intracranial hemangiopericytomas: outcomes after initial and repeat Gamma Knife surgery. J Neurosurg 112:133–139. doi:10.3171/2009.3.JNS0923
Rutkowski MJ, Bloch O, Jian BJ, Chen C, Sughrue ME, Tihan T, Barani IJ, Berger MS, McDermott MW, Parsa AT (2011) Management of recurrent intracranial hemangiopericytoma. J Clin Neurosci 18:1500–1504. doi:10.1016/j.jocn.2011.04.009
Ecker RD, Marsh WR, Pollock BE, Kurtkaya-Yapicier O, McClelland R, Scheithauer BW, Buckner JC (2003) Hemangiopericytoma in the central nervous system: treatment, pathological features, and long-term follow up in 38 patients. J Neurosurg 98:1182–1187. doi:10.3171/jns.2003.98.6.1182
Fountas KN, Kapsalaki E, Kassam M, Feltes CH, Dimopoulos VG, Robinson JS, Smith JR (2006) Management of intracranial meningeal hemangiopericytomas: outcome and experience. Neurosurg Rev 29:145–153. doi:10.1007/s10143-005-0001-9
Damodaran O, Robbins P, Knuckey N, Bynevelt M, Wong G, Lee G (2014) Primary intracranial haemangiopericytoma: comparison of survival outcomes and metastatic potential in WHO grade II and III variants. J Clin Neurosci 21:1310–1314. doi:10.1016/j.jocn.2013.11.026
Rajaram V, Brat DJ, Perry A (2004) Anaplastic meningioma versus meningeal hemangiopericytoma: immunohistochemical and genetic markers. Hum Pathol 35:1413–1418. doi:10.1016/j.humpath.2004.07.017
Trabelsi S, Mama N, Chourabi M, Mastouri MH, Ladib M, Popov S, Burford A, Mokni M, Tlili K, Krifa H, Jones C, Yacoubi MT, Saad A, Brahim DH (2015) Meningeal hemangiopericytomas and meningomas: a comparative immunohistochemical and genetic study. Asian Pac J Cancer Prev 16:6871–6876
Zhao P, Zhu T, Tang Q, Liu H, Zhu J, Zhang W (2015) Immunohistochemical and genetic markers to distinguish hemangiopericytoma and meningioma. Int J Clin Exp Med 8:3291–3299
Perry A, Scheithauer BW, Nascimento AG (1997) The immunophenotypic spectrum of meningeal hemangiopericytoma: a comparison with fibrous meningioma and solitary fibrous tumor of meninges. Am J Surg Pathol 21:1354–1360
Alawi F, Stratton D, Freedman PD (2001) Solitary fibrous tumor of the oral soft tissues: a clinicopathologic and immunohistochemical study of 16 cases. Am J Surg Pathol 25:900–910
Chaubal A, Paetau A, Zoltick P, Miettinen M (1994) CD34 immunoreactivity in nervous system tumors. Acta Neuropathol 88:454–458
Bouvier C, Metellus P, de Paula AM, Vasiljevic A, Jouvet A, Guyotat J, Mokhtari K, Varlet P, Dufour H, Figarella-Branger D (2012) Solitary fibrous tumors and hemangiopericytomas of the meninges: overlapping pathological features and common prognostic factors suggest the same spectrum of tumors. Brain Pathol 22:511–521. doi:10.1111/j.1750-3639.2011.00552.x
Macagno N, Figarella-Branger D, Mokthari K, Metellus P, Jouvet A, Vasiljevic A, Loundou A, Bouvier C (2015) Differential diagnosis of meningeal SFT-HPC and meningioma: which immunohistochemical markers should be used. Am J Surg Pathol. doi:10.1097/PAS.0000000000000526
Chmielecki J, Crago AM, Rosenberg M, O’Connor R, Walker SR, Ambrogio L, Auclair D, McKenna A, Heinrich MC, Frank DA, Meyerson M (2013) Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat Genet 45:131–132. doi:10.1038/ng.2522
Mohajeri A, Tayebwa J, Collin A, Nilsson J, Magnusson L, von Steyern FV, Brosjo O, Domanski HA, Larsson O, Sciot R, Debiec-Rychter M, Hornick JL, Mandahl N, Nord KH, Mertens F (2013) Comprehensive genetic analysis identifies a pathognomonic NAB2/STAT6 fusion gene, nonrandom secondary genomic imbalances, and a characteristic gene expression profile in solitary fibrous tumor. Genes Chromosom Cancer 52:873–886. doi:10.1002/gcc.22083
Robinson DR, Wu YM, Kalyana-Sundaram S, Cao X, Lonigro RJ, Sung YS, Chen CL, Zhang L, Wang R, Su F, Iyer MK, Roychowdhury S, Siddiqui J, Pienta KJ, Kunju LP, Talpaz M, Mosquera JM, Singer S, Schuetze SM, Antonescu CR, Chinnaiyan AM (2013) Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet 45:180–185. doi:10.1038/ng.2509
Fletcher CDM, Unni KK, Mertens F (2002) Pathology and genetics of tumours of soft tissue and bone. IARC, Lyon
Schweizer L, Koelsche C, Sahm F, Piro RM, Capper D, Reuss DE, Pusch S, Habel A, Meyer J, Gock T, Jones DT, Mawrin C, Schittenhelm J, Becker A, Heim S, Simon M, Herold-Mende C, Mechtersheimer G, Paulus W, Konig R, Wiestler OD, Pfister SM, von Deimling A (2013) Meningeal hemangiopericytoma and solitary fibrous tumors carry the NAB2-STAT6 fusion and can be diagnosed by nuclear expression of STAT6 protein. Acta Neuropathol 125:651–658. doi:10.1007/s00401-013-1117-6
Barthelmess S, Geddert H, Boltze C, Moskalev EA, Bieg M, Sirbu H, Brors B, Wiemann S, Hartmann A, Agaimy A, Haller F (2014) Solitary fibrous tumors/hemangiopericytomas with different variants of the NAB2-STAT6 gene fusion are characterized by specific histomorphology and distinct clinicopathological features. Am J Pathol 184:1209–1218. doi:10.1016/j.ajpath.2013.12.016
Koelsche C, Schweizer L, Renner M, Warth A, Jones DT, Sahm F, Reuss DE, Capper D, Knosel T, Schulz B, Petersen I, Ulrich A, Renker EK, Lehner B, Pfister SM, Schirmacher P, von Deimling A, Mechtersheimer G (2014) Nuclear relocation of STAT6 reliably predicts NAB2-STAT6 fusion for the diagnosis of solitary fibrous tumour. Histopathology 65:613–622. doi:10.1111/his.12431
Acknowledgments
The authors deeply thank to Dr. Rho (Jong-Lyel Roh, M.D., Ph.D., Department of Otolaryngology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea) for kindly reviewing the manuscript and advising on methods to improve it.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational Grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, + affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11060_2015_2030_MOESM1_ESM.docx
Kaplan-Meier analyses of overall survival (OS), cause-specific survival (CSS), recurrence-free survival (RFS), local control (LC), regional metastasis-free survival (r-MFS), and distant metastasis-free survival (d-MFS) in patients with WHO grade III HPCs. There was obvious trend toward prolonged OS and CSS in patients receiving PORT. (DOCX 342 kb)
Rights and permissions
About this article
Cite this article
Lee, E.J., Kim, J.H., Park, E.S. et al. The impact of postoperative radiation therapy on patterns of failure and survival improvement in patients with intracranial hemangiopericytoma. J Neurooncol 127, 181–190 (2016). https://doi.org/10.1007/s11060-015-2030-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-2030-8