Abstract
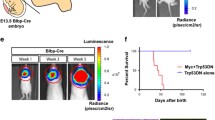
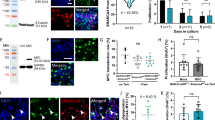
A highly aggressive subgroup of the pediatric brain tumor medulloblastoma is characterized by overexpression of the proto-oncogene c-Myc, which encodes a transcription factor that normally maintains neural progenitor cells in an undifferentiated, proliferating state during embryonic development. Myc-driven medulloblastomas typically show a large-cell anaplastic (LCA) histological pattern, in which tumor cells display large, round nuclei with prominent nucleoli. This subgroup of medulloblastoma is therapeutically challenging because it is associated with a high rate of metastatic dissemination, which is a powerful predictor of short patient survival times. Genetically engineered mouse models have revealed important insights into the pathogenesis of medulloblastoma and served as preclinical testing platforms for new therapies. Here we report a new mouse model of Myc-driven medulloblastoma, in which tumors arise in situ after retroviral transfer and expression of Myc in Nestin-expressing neural progenitor cells in the cerebella of newborn mice. Tumor induction required concomitant loss of Tp53 or overexpression of the antiapoptotic protein Bcl-2. Like Myc-driven medulloblastomas in humans, the tumors induced in mice by Myc + Bcl-2 and Myc − Tp53 showed LCA cytoarchitecture and a high rate of metastatic dissemination to the spine. The fact that Myc − Tp53 tumors arose only in Tp53 −/− mice, coupled with the inefficient germline transmission of the Tp53-null allele, made retroviral transfer of Myc + Bcl-2 a more practical method for generating LCA medulloblastomas. The high rate of spinal metastasis (87 % of brain tumor–bearing mice) will be an asset for testing new therapies that target the most lethal aspect of medulloblastoma.



Similar content being viewed by others
References
Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, Eberhart CG, Parsons DW, Rutkowski S, Gajjar A, Ellison DW, Lichter P, Gilbertson RJ, Pomeroy SL, Kool M, Pfister SM (2012) Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol 123:465–472. doi:10.1007/s00401-011-0922-z
Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, Bouffet E, Clifford SC, Hawkins CE, French P, Rutka JT, Pfister S, Taylor MD (2010) Medulloblastoma comprises four distinct molecular variants. J Clin Oncol 29:1408–1414. doi:10.1200/JCO.2009.27.4324
Ellison DW, Kocak M, Dalton J, Megahed H, Lusher ME, Ryan SL, Zhao W, Nicholson SL, Taylor RE, Bailey S, Clifford SC (2011) Definition of disease-risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J Clin Oncol 29:1400–1407. doi:10.1200/JCO.2010.30.2810
Northcott PA, Jones DT, Kool M, Robinson GW, Gilbertson RJ, Cho YJ, Pomeroy SL, Korshunov A, Lichter P, Taylor MD, Pfister SM (2012) Medulloblastomics: the end of the beginning. Nat Rev Cancer 12:818–834. doi:10.1038/nrc3410
Cohen MM Jr (2010) Hedgehog signaling update. Am J Med Genet A 152A:1875–1914. doi:10.1002/ajmg.a.32909
Rao G, Pedone CA, Coffin CM, Holland EC, Fults DW (2003) c-Myc enhances sonic hedgehog-induced medulloblastoma formation from nestin-expressing neural progenitors in mice. Neoplasia 5:198–204
Coon V, Laukert T, Pedone CA, Laterra J, Kim KJ, Fults DW (2010) Molecular therapy targeting Sonic hedgehog and hepatocyte growth factor signaling in a mouse model of medulloblastoma. Mol Cancer Ther 9:2627–2636. doi:10.1158/1535-7163.mct-10-0486
Smith K, Dalton S (2010) Myc transcription factors: key regulators behind establishment and maintenance of pluripotency. Regen Med 5:947–959. doi:10.2217/rme.10.79
Kawauchi D, Robinson G, Uziel T, Gibson P, Rehg J, Gao C, Finkelstein D, Qu C, Pounds S, Ellison DW, Gilbertson RJ, Roussel MF (2012) A mouse model of the most aggressive subgroup of human medulloblastoma. Cancer Cell 21:168–180. doi:10.1016/j.ccr.2011.12.023
Pei Y, Moore CE, Wang J, Tewari AK, Eroshkin A, Cho YJ, Witt H, Korshunov A, Read TA, Sun JL, Schmitt EM, Miller CR, Buckley AF, McLendon RE, Westbrook TF, Northcott PA, Taylor MD, Pfister SM, Febbo PG, Wechsler-Reya RJ (2012) An animal model of MYC-driven medulloblastoma. Cancer Cell 21:155–167. doi:10.1016/j.ccr.2011.12.021
Holland EC, Hively WP, DePinho RA, Varmus HE (1998) A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev 12:3675–3685
Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA (1994) Tumor spectrum analysis in p53-mutant mice. Curr Biol 4:1–7
Federspiel MJ, Bates P, Young JAT, Varmus HE, Hughes SH (1994) A system for tissue-specific gene targeting: transgenic mice susceptible to subgroup A avian leukosis virus-based retroviral vectors. Proc Natl Acad Sci USA 91:11241–11245
McCall TD, Pedone CA, Fults DW (2007) Apoptosis suppression by somatic cell transfer of Bcl-2 promotes Sonic hedgehog-dependent medulloblastoma formation in mice. Cancer Res 67:5179–5185. doi:10.1158/0008-5472.can-06-4177
Gregory MA, Qi Y, Hann SR (2003) Phosphorylation by glycogen synthase kinase-3 controls c-myc proteolysis and subnuclear localization. J Biol Chem 278:51606–51612
Welcker M, Orian A, Jin J, Grim JA, Harper JW, Eisenman RN, Clurman BE (2004) The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc Natl Acad Sci USA 101:9085–9090
Coffin CM, Braun JT, Wick MR, Dehner LP (1990) A clinicopathologic and immunohistochemical analysis of 53 cases of medulloblastomas with emphasis on synaptophysin expression. Mod Pathol 3:164–170
Mullen RJ, Buck CR, Smith AM (1992) NeuN, a neuronal specific nuclear protein in vertebrates. Development 116:201–211
Easter SS, Ross LS, Frankfurter A (1993) Initial tract formation in the mouse brain. J Neurosci 13:285–299
Zhukova N, Ramaswamy V, Remke M, Pfaff E, Shih DJ, Martin DC, Castelo-Branco P, Baskin B, Ray PN, Bouffet E, von Bueren AO, Jones DT, Northcott PA, Kool M, Sturm D, Pugh TJ, Pomeroy SL, Cho YJ, Pietsch T, Gessi M et al (2013) Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. J Clin Oncol 31:2927–2935. doi:10.1200/JCO.2012.48.5052
Green DR, Evan GI (2002) A matter of life and death. Cancer Cell 1:19–30
Junttila MR, Evan GI (2009) p53: a Jack of all trades but master of none. Nat Rev Cancer 9:821–829. doi:10.1038/nrc2728
Delbridge AR, Strasser A (2015) The BCL-2 protein family, BH3-mimetics and cancer therapy. Cell Death Differ 22:1071–1080. doi:10.1038/cdd.2015.50
Schüller U, Schober F, Kretzschmar HA, Herms J (2004) Bcl-2 expression inversely correlates with tumour cell differentiation in medulloblastoma. Neuropathol Appl Neurobiol 30:513–521
Czabotar PE, Lessene G, Strasser A, Adams JM (2014) Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol 15:49–63. doi:10.1038/nrm3722
Cho YJ, Tsherniak A, Tamayo P, Santagata S, Ligon A, Greulich H, Berhoukim R, Amani V, Goumnerova L, Eberhart CG, Lau CC, Olson JM, Gilbertson RJ, Gajjar A, Delattre O, Kool M, Ligon K, Meyerson M, Mesirov JP, Pomeroy SL (2011) Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol 29:1424–1430. doi:10.1200/JCO.2010.28.5148
Holland EC, Varmus HE (1998) Basic fibroblast growth factor induces cell migration and proliferation after glia-specific gene transfer in mice. Proc Natl Acad Sci USA 95:1218–1223
von Werder A, Seidler B, Schmid RM, Schneider G, Saur D (2012) Production of avian retroviruses and tissue-specific somatic retroviral gene transfer in vivo using the RCAS/TVA system. Nat Protoc 7:1167–1183. doi:10.1038/nprot.2012.060
Kasuga C, Nakahara Y, Ueda S, Hawkins C, Taylor MD, Smith CA, Rutka JT (2008) Expression of MAGE and GAGE genes in medulloblastoma and modulation of resistance to chemotherapy. Lab Investig J Neurosurg Pediatr 1:305–313. doi:10.3171/PED/2008/1/4/305
Oba-Shinjo SM, Caballero OL, Jungbluth AA, Rosemberg S, Old LJ, Simpson AJ, Marie SK (2008) Cancer-testis (CT) antigen expression in medulloblastoma. Cancer Immun Arch 8:7
Sonabend AM, Ogden AT, Maier LM, Anderson DE, Canoll P, Bruce JN, Anderson RC (2012) Medulloblasoma: challenges for effective immunotherapy. J Neurooncol 108:1–10. doi:10.1007/s11060-011-0776-1
Doucette T, Yang Y, Zhang W, Fuller GN, Suki D, Fults DW, Rao G (2011) Bcl-2 promotes malignant progression in a PDGF-B-dependent murine model of oligodendroglioma. Int J Cancer 129:2093–2103. doi:10.1002/ijc.25869
Laulier C, Lopez BS (2012) The secret life of Bcl-2: apoptosis-independent inhibition of DNA repair by Bcl-2 family members. Mutat Res 751:247–257. doi:10.1016/j.mrrev.2012.05.002
Othman RT, Kimishi I, Bradshaw TD, Storer LC, Korshunov A, Pfister SM, Grundy RG, Kerr ID, Coyle B (2014) Overcoming multiple drug resistance mechanisms in medulloblastoma. Acta Neuropathol Commun 2:57. doi:10.1186/2051-5960-2-57
van Riggelen J, Yetil A, Felsher DW (2010) MYC as a regulator of ribosome biogenesis and protein synthesis. Nat Rev Cancer 10:301–309. doi:10.1038/nrc2819
Pierce SB, Yost C, Britton JS, Loo LW, Flynn EM, Edgar BA, Eisenman RN (2004) dMyc is required for larval growth and endoreplication in Drosophila. Development 131:2317–2327. doi:10.1242/dev.01108
Kim S, Li Q, Dang CV, Lee LA (2000) Induction of ribosomal genes and hepatocyte hypertrophy by adenovirus-mediated expression of c-Myc in vivo. Proc Natl Acad Sci USA 97:11198–11202. doi:10.1073/pnas.200372597
Iritani BM, Eisenman RN (1999) c-Myc enhances protein synthesis and cell size during B lymphocyte development. Proc Natl Acad Sci USA 96:13180–13185
Momota H, Shih AH, Edgar MA, Holland EC (2008) c-Myc and beta-catenin cooperate with loss of p53 to generate multiple members of the primitive neuroectodermal tumor family in mice. Oncogene 27:4392–4401. doi:10.1038/onc.2008.81
Shakhova O, Leung C, van Montfort E, Berns A, Marino S (2006) Lack of Rb and p53 delays cerebellar development and predisposes to large cell anaplastic medulloblastoma through amplification of N-Myc and Ptch2. Cancer Res 66:5190–5200. doi:10.1158/0008-5472.CAN-05-3545
Shih DJ, Northcott PA, Remke M, Korshunov A, Ramaswamy V, Kool M, Luu B, Yao Y, Wang X, Dubuc AM, Garzia L, Peacock J, Mack SC, Wu X, Rolider A, Morrissy AS, Cavalli FM, Jones DT, Zitterbart K, Faria CC et al (2014) Cytogenetic prognostication within medulloblastoma subgroups. J Clin Oncol 32:886–896. doi:10.1200/JCO.2013.50.9539
Stearns D, Chaudhry A, Abel TW, Burger PC, Dang CV, Eberhart CG (2006) c-myc overexpression causes anaplasia in medulloblastoma. Cancer Res 66:673–681. doi:10.1158/0008-5472.CAN-05-1580
Jenkins NC, Kalra RR, Dubuc A, Sivakumar W, Pedone CA, Wu X, Taylor MD, Fults DW (2014) Genetic drivers of metastatic dissemination in sonic hedgehog medulloblastoma. Acta Neuropathol Commun 2:85. doi:10.1186/s40478-014-0085-y
Acknowledgments
The authors thank Kristin Kraus (University of Utah) for editorial assistance. This work was supported by Grants from the National Institutes of Health (R01CA18622 to DWF and K08NS070928 to GR) and the Huntsman Cancer Institute of the University of Utah (P30CA042014 to DF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11060_2015_1985_MOESM1_ESM.tif
Supplementary Fig. 1. Comparative expression of Myc and prosurvival Bcl-2 family member genes in human Group 3 medulloblastomas. The color-coded Z-score for each tumor specimen is the number of standard deviations that the expression level of a gene is shifted above (red) or below (blue) the mean. Z-scores are shown for a discovery cohort (n = 46) and an independent validation cohort (n = 51). Purple bars below indicate tumors in which expression of both Myc and Bcl-2 was increased. Supplementary material 1(TIFF 1171 kb)
11060_2015_1985_MOESM2_ESM.tif
Supplementary Fig. 2. Kaplan–Meier survival analysis of mice after retroviral transfer of Shh and Myc + Bcl-2. The mice were injected with RCAS-SHH or RCAS-Myc and RCAS-Bcl-2 on day zero and sacrificed at the indicated time points. Supplementary material 2 (TIFF 8237 kb)
Rights and permissions
About this article
Cite this article
Jenkins, N.C., Rao, G., Eberhart, C.G. et al. Somatic cell transfer of c-Myc and Bcl-2 induces large-cell anaplastic medulloblastomas in mice. J Neurooncol 126, 415–424 (2016). https://doi.org/10.1007/s11060-015-1985-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1985-9