Abstract
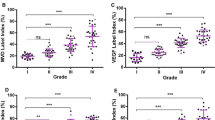
Roundabout4 (Robo4), a new member of Robo proteins family, is specifically expressed in endothelial cells. Recent studies have indicated that Robo4 could regulate tumor angiogenesis and vascular permeability. However, the role and function of Robo4 are not well understood. This study was performed to investigate the expression of Robo4 in primary glioma patients, and thus to determine the association of Robo4 expression with microvessel density and survival of glioma patients. In this study, real-time PCR and immunohistochemistry were performed to examine the mRNA level and protein expression of Robo4 in both 43 cases of glioma samples and 10 cases of normal brain tissue samples. The results demonstrated that Robo4 was significantly up-regulated in glioma tissues compared with normal brain tissues. In addition, double immunofluorescent staining revealed that Robo4 expression co-localized with CD34 expression in the vessel of glioma tissues. The expression of Robo4 positively correlated with patients’ age (P = 0.0139) and glioma grade (P < 0.0001). A linear correlation was observed between the relative mRNA expression of Robo4 values and corresponding microvessel density values (r = 0.9735, P < 0.0001). Kaplan–Meier analysis and log-rank test result showed that the overall survival of patients with Robo4 high expression was significantly shorter than that of patients with Robo4 low expression (P < 0.001). The results of present study verify that overexpression of Robo4 is related to poor prognosis of primary gliomas patients through correlating with microvessel density.



Similar content being viewed by others
References
Louis DN (2006) Molecular pathology of malignant gliomas. Annu Rev Pathol 1:97–117
Demuth T, Berens ME (2004) Molecular mechanisms of glioma cell migration and invasion. J Neurooncol 70:217–228
Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT (2007) Angiogenesis in brain tumours. Nat Rev Neurosci 8:610–622
Chi AS, Sorensen AG, Jain RK, Batchelor TT (2009) Angiogenesis as a therapeutic target in malignant gliomas. Oncologist 14:621–636
Plate KH, Risau W (1995) Angiogenesis in malignant gliomas. Glia 15:339–347
Takano S, Kamiyama H, Tsuboi K, Matsumura A (2004) Angiogenesis and antiangiogenic therapy for malignant gliomas. Brain Tumor Pathol 21:69–73
Leon SP, Folkerth RD, Black PM (1996) Microvessel density is a prognostic indicator for patients with astroglial brain tumors. Cancer 77:362–372
Liu XM, Zhang QP, Mu YG, Zhang XH, Sai K, Pang JC, Ng HK, Chen ZP (2011) Clinical significance of vasculogenic mimicry in human gliomas. J Neurooncol 105:173–179
Udani VM, Santarelli JG, Yung YC, Wagers AJ, Cheshier SH, Weissman IL, Tse V (2005) Hematopoietic stem cells give rise to perivascular endothelial-like cells during brain tumor angiogenesis. Stem Cells Dev 14:478–486
Gaiser T, Becker MR, Meyer J, Habel A, Siegelin MD (2009) p53-mediated inhibition of angiogenesis in diffuse low-grade astrocytomas. Neurochem Int 54:458–463
Bodey B, Bodey BJ, Siegel SE, Kaiser HE (1998) Upregulation of endoglin (CD105) expression during childhood brain tumor-related angiogenesis. Anti-angiogenic therapy. Anticancer Res 18:1485–1500
Huminiecki L, Gorn M, Suchting S, Poulsom R, Bicknell R (2002) Magic roundabout is a new member of the roundabout receptor family that is endothelial specific and expressed at sites of active angiogenesis. Genomics 79:547–552
Legg JA, Herbert JM, Clissold P, Bicknell R (2008) Slits and Roundabouts in cancer, tumour angiogenesis and endothelial cell migration. Angiogenesis 11:13–21
Seth P, Lin Y, Hanai J, Shivalingappa V, Duyao MP, Sukhatme VP (2005) Magic roundabout, a tumor endothelial marker: expression and signaling. Biochem Biophys Res Commun 332:533–541
Bedell VM, Yeo SY, Park KW, Chung J, Seth P, Shivalingappa V, Zhao J, Obara T, Sukhatme VP, Drummond IA, Li DY, Ramchandran R (2005) Roundabout4 is essential for angiogenesis in vivo. Proc Natl Acad Sci USA 102:6373–6378
Weidner N, Semple JP, Welch WR, Folkman J (1991) Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N Engl J Med 324:1–8
Sinha S, Singh RK, Bhattacharya N, Mukherjee N, Ghosh S, Alam N, Roy A, Roychoudhury S, Panda CK (2011) Frequent alterations of LOH11CR2A, PIG8 and CHEK1 genes at chromosomal 11q24.1-24.2 region in breast carcinoma: clinical and prognostic implications. Mol Oncol 5:454–464
Ohgaki H, Kleihues P (2005) Epidemiology and etiology of gliomas. Acta Neuropathol 109:93–108
Fischer I, Gagner JP, Law M, Newcomb EW, Zagzag D (2005) Angiogenesis in gliomas: biology and molecular pathophysiology. Brain Pathol 15:297–310
Park KW, Morrison CM, Sorensen LK, Jones CA, Rao Y, Chien CB, Wu JY, Urness LD, Li DY (2003) Robo4 is a vascular-specific receptor that inhibits endothelial migration. Dev Biol 261:251–267
Suchting S, Heal P, Tahtis K, Stewart LM, Bicknell R (2005) Soluble Robo4 receptor inhibits in vivo angiogenesis and endothelial cell migration. FASEB J 19:121–123
Andrews WD, Barber M, Parnavelas JG (2007) Slit-Robo interactions during cortical development. J Anat 211:188–198
Shao Y, Zhou Y, Hou Y, He J, Hu L, Zhang Y, Jiang Y, Lu W, Liu H (2014) Prognostic implications of SLIT and ROBO1 expression in Gallbladder cancer. Cell Biochem Biophys 70:747–758
Mano Y, Aishima S, Fukuhara T, Tanaka Y, Kubo Y, Motomura T, Toshima T, Iguchi T, Shirabe K, Maehara Y, Oda Y (2013) Decreased roundabout 1 expression promotes development of intrahepatic cholangiocarcinoma. Hum Pathol 44:2419–2426
Ghosh S, Ghosh A, Maiti GP, Alam N, Roy A, Roychoudhury S, Panda CK (2009) Alterations of ROBO1/DUTT1 and ROBO2 loci in early dysplastic lesions of head and neck: clinical and prognostic implications. Hum Genet 125:189–198
Mitra S, Mazumder-Indra D, Mondal RK, Basu PS, Roy A, Roychoudhury S, Panda CK (2012) Inactivation of SLIT2-ROBO1/2 pathway in premalignant lesions of uterine cervix: clinical and prognostic significances. PLoS One 7:e38342
Acknowledgments
This work is supported by grants from the Natural Science Foundation of China (nos. 81172197, 81171131, 81272564, 81272795, 81372484, and 81372682), Shenyang Science and Technology Plan Projects (nos. F13-318-1-16, F13-318-1-19 and F13-220-9-15), and Outstanding Scientific Fund of Shengjing Hospital (no. 201304).
Conflict of interest
The authors disclose that no potential conflicts of interest exist.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11060_2015_1780_MOESM1_ESM.tif
Supplementary Fig. 1 Mean optical densities of Robo4 (a) and CD-34 (b) are shown. Values are mean ± SD. Arrows show capillaries. **P < 0.01 versus normal brain tissues group. Supplementary material 1 (TIFF 3708 kb)
11060_2015_1780_MOESM2_ESM.tif
Supplementary Fig. 2 Immunohistochemistry analysis of VWF protein in primary glioma and normal brain tissues (magnification ×400; scale bar 25 μm). Supplementary material 2 (TIFF 1516 kb)
11060_2015_1780_MOESM3_ESM.tif
Supplementary Fig. 3 Immunofluorescence staining of Robo4 in primary glioma and normal brain tissues. Mean optical densities of Robo4 (a) and CD-34 (b) are shown. Values are mean ± SD. Arrows show capillaries. **P < 0.01 versus normal brain tissues group. Supplementary material 3 (TIFF 8445 kb)
Rights and permissions
About this article
Cite this article
Cai, H., Xue, Y., Liu, W. et al. Overexpression of Roundabout4 predicts poor prognosis of primary glioma patients via correlating with microvessel density. J Neurooncol 123, 161–169 (2015). https://doi.org/10.1007/s11060-015-1780-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1780-7