Abstract
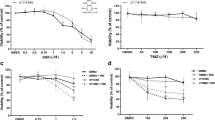
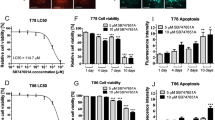
The poor prognosis of patients with glioblastoma fuels the search for more effective therapeutic compounds. We previously hypothesised that the neuroleptic olanzapine may enhance antineoplastic effects of temozolomide the standard chemotherapeutic agent used in this disease. This study tested this hypothesis. The anti-proliferative effect of olanzapine was examined by MTT assays and cell count analysis. Soft-agar assays were performed to examine colony-forming ability. In addition, the inhibitory effect of olanzapine on the migratory capacity of U87MG and A172 cells was analyzed by Transwell® assays. Moreover, staining for annexin V/propidium iodide or carboxyfluorescein succinimidyl ester was performed prior to flow cytometric analysis in order to better understand the subjacent cellular mechanism. Our initial hypothesis that olanzapine may enhance temozolomide’s anti-tumor activity could be confirmed in U87MG and A172 glioblastoma cell lines. Moreover, treatment with olanzapine alone resulted in a marked anti-proliferative effect on U87MG, A172 and two glioma stem-like cells with IC50 values ranging from 25 to 79.9 µM. In U87MG cells, anchorage-independent growth was dose-dependently inhibited. In A172 cells, migration was also shown to be inhibited in a dose-dependent manner. In addition, olanzapine was shown to exert a cell line-dependent pleomorphism with respect to the induction of apoptosis, necrosis and/or cytostasis. Our data show that the neuroleptic olanzapine enhances the anti-tumor activity of temozolomide against glioblastoma cell lines. Moreover, this is the first study to show that olanzapine provides on its own anti-cancer activity in glioblastoma and thus may have potential for repurposing.






Similar content being viewed by others
References
Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS (2013) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol 15(Suppl 2):ii1–ii56
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Kast RE, Karpel-Massler G, Halatsch ME (2011) Can the therapeutic effects of temozolomide be potentiated by stimulating AMP-activated protein kinase with olanzepine and metformin? Br J Pharmacol 164(5):1393–1396
Zhang WB, Wang Z, Shu F, Jin YH, Liu HY, Wang QJ, Yang Y (2010) Activation of AMP-activated protein kinase by temozolomide contributes to apoptosis in glioblastoma cells via p53 activation and mTORC1 inhibition. J Biol Chem 285(52):40461–40471
Kim SF, Huang AS, Snowman AM, Teuscher C, Snyder SH (2007) From the cover: antipsychotic drug-induced weight gain mediated by histamine H1 receptor-linked activation of hypothalamic AMP-kinase. Proc Natl Acad Sci USA 104(9):3456–3459
Buckley PF (2005) Olanzapine: a critical review of recent literature. Expert Opin Pharmacother 6(12):2077–2089
Frye MA (2011) Clinical practice. Bipolar disorder—a focus on depression. N Engl J Med 364(1):51–59
Dipple HC (1999) The use of olanzapine for movement disorder in Huntington’s disease: a first case report. J Neurol Neurosurg Psychiatry 67(1):123–124
Grove VE Jr, Quintanilla J, DeVaney GT (2000) Improvement of Huntington’s disease with olanzapine and valproate. N Engl J Med 343(13):973–974
Awad AG, Voruganti LN (2004) Impact of atypical antipsychotics on quality of life in patients with schizophrenia. CNS Drugs 18(13):877–893
Eder U, Mangweth B, Ebenbichler C, Weiss E, Hofer A, Hummer M, Kemmler G, Lechleitner M, Fleischhacker WW (2001) Association of olanzapine-induced weight gain with an increase in body fat. Am J Psychiatry 158(10):1719–1722
Kapur S, Zipursky RB, Remington G, Jones C, DaSilva J, Wilson AA, Houle S (1998) 5-HT2 and D2 receptor occupancy of olanzapine in schizophrenia: a PET investigation. Am J Psychiatry 155(7):921–928
Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, Moore NA, Tye NC, Seeman P, Wong DT (1996) Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology 14(2):87–96
Tauscher J, Jones C, Remington G, Zipursky RB, Kapur S (2002) Significant dissociation of brain and plasma kinetics with antipsychotics. Mol Psychiatry 7(3):317–321
ZYPREXA: EPAR-product information. http://www.emaeuropaeu/ema/indexjsp?curl=pages/medicines/human/medicines/000115/human_med_001189jsp&murl=menus/medicines/medicinesjsp&mid=WC0b01ac058001d125. (Last Accessed 17 Dec 2013)
Ring BJ, Catlow J, Lindsay TJ, Gillespie T, Roskos LK, Cerimele BJ, Swanson SP, Hamman MA, Wrighton SA (1996) Identification of the human cytochromes P450 responsible for the in vitro formation of the major oxidative metabolites of the antipsychotic agent olanzapine. J Pharmacol Exp Ther 276(2):658–666
Kast RE, Foley KF (2007) Cancer chemotherapy and cachexia: mirtazapine and olanzapine are 5-HT3 antagonists with good antinausea effects. Eur J Cancer Care (Engl) 16(4):351–354
Khojainova N, Santiago-Palma J, Kornick C, Breitbart W, Gonzales GR (2002) Olanzapine in the management of cancer pain. J Pain Symptom Manage 23(4):346–350
Navari RM (2014) Olanzapine for the prevention and treatment of chronic nausea and chemotherapy-induced nausea and vomiting. Eur J Pharmacol 722:180–186
Navari RM, Nagy CK, Gray SE (2013) The use of olanzapine versus metoclopramide for the treatment of breakthrough chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy. Support Care Cancer 21(6):1655–1663
Prommer E (2013) Olanzapine: palliative medicine update. Am J Hosp Palliat Care 30(1):75–82
Westhoff MA, Zhou S, Nonnenmacher L, Karpel-Massler G, Jennewein C, Schneider M, Halatsch ME, Carragher NO, Baumann B, Krause A, Simmet T, Bachem MG, Wirtz CR, Debatin KM (2013) Inhibition of NF-κB signaling ablates the invasive phenotype of glioblastoma. Mol Cancer Res 11(12):1611–1623
Wlodkowic D, Telford W, Skommer J, Darzynkiewicz Z (2011) Apoptosis and beyond: cytometry in studies of programmed cell death. Methods Cell Biol 103:55–98
Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, Cahill DP, Nahed BV, Curry WT, Martuza RL, Louis DN, Rozenblatt-Rosen O, Suvà ML, Regev A, Bernstein BE (2014) Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344:1396–1401
Liang J, Mills GB (2013) AMPK: a contextual oncogene or tumor suppressor? Cancer Res 73(10):2929–2935
Jeon S-M, Chandel NS, Hay N (2012) AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 485(7400):661–665
Ferno J, Skrede J, Vik-Mo AO, Havik B, Steen VM (2006) Drug-induced activation of SREBP-controlled lipogenic gene expression in CNS-related cell lines: marked differences between various antipsychotic drugs. BMC Neurosci 7:69–80
Krzystanek M, Krzystanek E, Trzeciak HI, Malecki A, Krupka-Matuszczyk I, Janas-Kozik M, Rybakowski JK (2013) Effects of olanzapine and paroxetine on phospholipase D activity in the rat brain. Pharmacol Rep 65:724–729
Exton JH (1999) Regulation of phospholipase D. Biochim Biophys Acta 1439:121–133
Klein J (2005) Functions and pathophysiological roles of phospholipase D in the brain. J Neurochem 94:1473–1487
Foster DA, Xu L (2003) Phospholipase D in cell proliferation and cancer. Mol Cancer Res 1:789–800
Min DS, Kwon TK, Park W-S, Chang J-S, Park S-K, Ahn B-H, Ryoo ZY, Lee YH, Lee YS, Rhie D-J, Yoon S-H, Hahn SJ, Kim M-S, Jo Y-H (2001) Neoplastic transformation and tumorigenesis associated with expression of phospholipase D isozymes in cultured murine fibroblasts. Carcinogenesis 22:1641–1647
Park MH, Ahn BH, Hong YK, Min DS (2009) Overexpression of phospholipase D enhances matrix metalloproteinase-2 expression and glioma cell invasion via protein kinase C and protein kinase A/NF-kappaB/Sp1-mediated signaling pathways. Carcinogenesis 30:356–365
Bruntz RC, Taylor HE, Lindsley CW, Brown HA (2013) Phospholipase D2 mediates survival signaling through direct regulation of Akt in glioblastoma cells. J Biol Chem 289(2):600–616
Kim NR, Park SW, Lee JG, Kim YH (2008) Protective effects of olanzapine and haloperidol on serum withdrawal-induced apoptosis in SH-SY5Y cells. Prog Neuropsychopharmacol Biol Psychiatry 32:633–642
Wakade CG, Mahadik SP, Waller JL, Chiu F-C (2002) Atypical neuroleptics stimulate neurogenesis in adult rat brain. J Neurosci Res 69:72–79
Wilcock C, Bailey CJ (1994) Accumulation of metformin by tissues of the normal and diabetic mouse. Xenobiotica 24:49–57
Kornhuber J, Schultz A, Wiltfang J, Meineke I, Gleiter CH, Zöchling R, Boissl K-W, Leblhuber F, Riederer P (1999) Persistence of haloperidol in human brain tissue. Am J Psychiatry 156:885–890
Kast RE, Bookvar JA, Brüning A, Cappello F, Chang WW, Cvek B, Dou QP, Duenas-Gonzalez A, Efferth T, Focosi D, Ghaffari SH, Karpel-Massler G, Ketola K, Khoshnevisan A, Keizman D, Magné N, Marosi C, McDonald K, Munoz M, Paranjpe A, Pourgholami MH, Sardi I, Sella A, Srivenugopal KS, Tuccori M, Wang W, Wirtz CR, Halatsch M-E (2013) A conceptually new treatment approach for relapsed glioblastoma: coordinated undermining of survival paths with nine repurpused drugs (CUSP9) by the International Initiative for Accelerated Improvement of Glioblastoma Care. Oncotarget 4:502–530
Acknowledgments
Georg Karpel-Massler was supported by a young investigator’s grant provided by the Faculty of Medicine of the University of Ulm and by a Dr. Mildred Scheel postdoctoral scholarship of the German Cancer Aid. Markus D. Siegelin was supported by NIH Grant K08NS083732 (NINDS). We thank Angelika Vollmer, Andrea Dittrich and Susanne Baumgart for providing excellent technical assistance.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karpel-Massler, G., Kast, R.E., Westhoff, MA. et al. Olanzapine inhibits proliferation, migration and anchorage-independent growth in human glioblastoma cell lines and enhances temozolomide’s antiproliferative effect. J Neurooncol 122, 21–33 (2015). https://doi.org/10.1007/s11060-014-1688-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1688-7