ABSTRACT
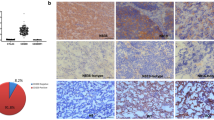
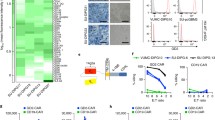
Immunobiology of medulloblastoma (MB), the most common malignant brain tumor in children, is poorly understood. Although tumor cells in some MBs were recently shown to express CD1d and be susceptible to Vα24-invariant natural killer T (NKT)-cell cytotoxicity, the clinical relevance of CD1d expression in MB patients remains unknown. We investigated the expression of CD1d in pediatric MBs and correlated with molecular and clinical characteristics. Specifically, we explored if NKT cell therapy can be targeted at a subset of pediatric MBs with poorer prognosis. Particularly, infantile MBs have a worse outcome because radiotherapy is delayed to avoid neurocognitive sequelae. Immunohistochemistry for CD1d was performed on a screening set of 38 primary pediatric MBs. Gene expression of the membrane form of M2 macrophage marker, CD163, was studied in an expanded cohort of 60 tumors. Outcome data was collected prospectively. Thirteen of 38 MBs (34.2 %) expressed CD1d on immunohistochemistry. CD1d was expressed mainly on MB tumor cells, and on some tumor-associated macrophages. Majority (18/22, 82 %) of non sonic-hedgehog/Wingless-activated MBs (group 3 and 4) were CD1d-negative (p = 0.05). A subset of infantile MBs (4/9, 44.4 %) expressed CD1d. Macrophages infiltrating MB expressed CD163 apart from CD1d. Molecular subtypes demonstrated statistical differences in CD163 expression, SHH-tumors were the most enriched (p = 0.006). Molecular and clinical subtypes of pediatric MB exhibit distinct differences in CD1d expression, which have important therapeutic implications. High CD1d expression in infantile MBs offers potential new immunotherapeutic treatment with NKT cell therapy in infants, where treatment is suboptimal due delayed radiotherapy.



Similar content being viewed by others
REFERENCES
Stastny MJ, Brown CE, Ruel C et al (2007) Medulloblastomas expressing IL13Ralpha2 are targets for IL13-zetakine+ cytolytic T cells. J Pediatr Hematol Oncol 29(10):669–677
Ahmed N, Ratnayake M, Savoldo B et al (2007) Regression of experimental medulloblastoma following transfer of HER2-specific T cells. Cancer Res 67(12):5957–5964
Liu D, Song L, Brawley VS et al (2013) Medulloblastoma expresses CD1d and can be targeted for immunotherapy with NKT cells. Clin Immunol 149(1):55–64
Kronenberg M, Gapin L (2002) The unconventional lifestyle of NKT cells. Nat Rev Immunol 2(8):557–568
Song L, Asgharzadeh S, Salo J et al (2009) Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J Clin Invest 119(6):1524–1536
Tachibana T, Onodera H, Tsuruyama T et al (2005) Increased intratumor Valpha24-positive natural killer T cells: a prognostic factor for primary colorectal carcinomas. Clin Cancer Res 11(20):7322–7327
Godfrey DI, Berzins SP (2007) Control points in NKT-cell development. Nat Rev Immunol 7:505–518
Taniguchi M, Seino K, Nakayama T (2003) The NKT cell system: bridging innate and acquired immunity. Nat Immunol 4(12):1164–1165
Burrows PD, Kroenberg M, Taniguchi M (2009) NKT cells turn ten. Nat Immunol 10(7):669–671
Gapin L (2008) The making of NKT cells. Nat Immunol 9(9):1009–1011
Terabe M, Berzofsky JA (2007) NKT cells in immunoregulation of tumor immunity: a new immunoregulatory axis. Trends Immunol 28(11):491–496
Ambrosino E, Terabe M, Halder RC et al (2007) Cross-regulation between Type I and Type II NKT cells in regulating tumor immunity: a new immunoregulatory axis. J Immunol 179:5126–5136
Loza MJ, Metelitsa LS, Perussia B (2002) NKT and T cells: coordinate regulation of NK-like phenotype and cytokine production. Eur J Immunol 32:3453–3462
Baev DV, Peng X, Song L et al (2004) Distinct homeostatic requirements of CD4+ and CD4− subsets of Vα24-invariant natural killer T cells in humans. Blood 104(13):4150–4158
Bendelac A, Savage PB, Teyton L (2007) The biology of NKT cells. Annu Rev Immunol 25:297–336
Li X, Shiratsuchi T, Chen G (2009) Invariant TCR rather than CD1d shapes the preferential activities of c-glycoside analogues against human versus murine invariant NKT cells. J Immunol 183:4415–4421
Metelitsa LS, Weinberg KI, Emanuel PD et al (2003) Expression of CD1d by myelomonocytic leukemias provides a target for cytotoxic NKT cells. Leukemia 17:1068–1077
Metelitsa LS, Naidenko OV, Kant A et al (2001) Human NKT cells mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. J Immunol 167:3114–3122
Godfrey DI, McCluskey J, Rossjohn J (2005) CD1d antigen presentation: treats for NKT cells. Nat Immunol 6(8):754–756
Natalie AB, Kwok SW, Lars KN et al (2007) CD1d–lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature 448(5):44–49
Mulhern RK, Horowitz ME, Kovnar EH et al (1989) Neurodevelopmental status of infants and young children treated for brain tumors with preirradiation chemotherapy. J Clin Oncol 7:1660–1666
Jenkin D, Danjoux C, Greenberg M (1998) Subsequent quality of life for children irradiated for a brain tumor before age four years. Med Pediatr Oncol 31:506–511
Kiltie AE, Lashford LS, Gattamaneni HR (1997) Survival and late effects in medulloblastoma patients treated with craniospinal irradiation under three years old. Med Pediatr Oncol 28:348–354
Hoppe-Hirsch E, Brunet L, Laroussinie F et al (1995) Intellectual outcome in children with malignant tumors of the posterior fossa: influence of the field of irradiation and quality of surgery. Childs Nerv Syst 11:340–346
Palmer SL, Goloubeva O, Reddick WE et al (2001) Patterns of intellectual development among survivors of pediatric medulloblastoma: a longitudinal analysis. J Clin Oncol 19:2302–2308
Teo WY, Shen J, Su JM et al (2013) Implications of tumor location on subtypes of medulloblastoma. Pediatr Blood Cancer 60(9):1408–1410
Clarkson DB, Fan YA, Joe H (1993) A remark on algorithm 643: FEXACT: an algorithm for performing Fisher’s Exact Test in r x c contingency tables. ACM Trans on Math Softw 19:484–488
Kaplan EL, Meier P (1958) Nonparametric evaluation from incomplete observations. J Am Stat Assoc 53:457–481
Taylor MD, Northcott PA, Korshunov A et al (2012) Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol 123:465–472
Van GH, Delputte P, Nauwynck H (2010) Scavenger receptor CD163, a Jack-of-all-trades and potential target for cell-directed therapy. Mol Immunol 47:1650–1660
Mantovani A, Allavena P, Sica A et al (2008) Cancer-related inflammation. Nature 454:436–444
Sica A, Larghi P, Mancino A et al (2008) Macrophage polarization in tumour progression. Semin Cancer Bio 18:349–355
Sica A, Bronte V (2007) Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Invest 117:1155–1166
Shu Q, Wong KK, Su JM et al (2008) Direct orthotopic transplantation of fresh surgical specimen preserves CD133+ tumor cells in clinically relevant mouse models of medulloblastoma and glioma. Stem Cells 26:1414–1424
Kaatsch P, Rickert CH, Kuehl J et al (2001) Population-based epidemiologic data on brain tumors in German children. Cancer 92:3155–3164
MacDonald TJ (2008) Aggressive infantile embryonal tumors. Child Neurol 23:1195–1203
Johnston DL, Keene D, Bartels U et al (2009) Medulloblastoma in children under the age of three years: a retrospective Canadian review. J Neurooncol 94:51–56
Bouffet E (2010) Medulloblastoma in infants the critical issues of the dilemma. Curr Oncol 17(3):2–3
Rutkowski S, Cohen B, Finlay J et al (2010) Medulloblastoma in young children. Pediatr Blood Cancer 54:635–637
White L, Kellie S, Gray E et al (1998) Postoperative chemotherapy in children less than 4 years of age with malignant brain tumors: promising initial response to a VETOPEC-based regimen. J Pediatr Hematol Oncol 20:125–130
Ater JL, van Eys J, Woo SY et al (1997) MOPP chemotherapy without irradiation as primary postsurgical therapy for brain tumors in infants and young children. J Neurooncol 32:243–252
Baram TZ, van Eys J, Dowell RE et al (1987) Survival and neurologic outcome of infants with medulloblastoma treated with surgery and MOPP chemotherapy: a preliminary report. Cancer 60:173–177
Lafay-Cousin L, Bouffet E, Hawkins C et al (2009) Impact of radiation avoidance on survival and neurocognitive outcome in infant medulloblastoma. Curr Oncol 16(6):21–28
Grundy RG, Wilne SH, Robinson KJ et al (2010) Primary postoperative chemotherapy without radiotherapy for treatment of brain tumours other than ependymoma in children under 3 years: results of the first UKCCSG/SIOP CNS 9204 trial. Eur J of Cancer 46:120–133
Rutkowski S, Bode U, Deinlein F et al (2005) Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med 352:978–986
Dhodapkar KM, Cirignano B, Chamian F et al (2004) Invariant natural killer T cells are preserved in patients with glioma and exhibit antitumor activity following dendritic cell-mediated expansion. Int J Cancer 109:893–899
Kronenberg M (2005) Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol 23:877–900
Swann J, Crowe NY, Hayakawa Y et al (2004) Regulation of antitumour immunity by CD1d-restricted NKT cells. Immunol Cell Biol 82:323–331
Molling JW, Langius JA, Langendijk JA et al (2007) Low levels of circulating invariant natural killer T cells predict poor clinical outcome in patients with head and neck squamous cell carcinoma. J Clin Oncol 25:862–868
Asgharzadeh S, Salo JA, Ji L et al (2012) Clinical significance of tumor-associated inflammatory cells in metastatic neuroblastoma. J Clin Oncol 30(28):3525–3532
Acknowledgments
Supported by NIH Grant CA109467 and Grants from the Gillson Longenbaugh Foundation, John S. Dunn Research Foundation, the Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation and National Medical Research Council Research Fellowship (Singapore).
Conflict of interests
All the authors declared no competing or conflict of interests, or financial disclosures that are relevant to the subject matter under consideration in this article.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Wan-Yee Teo and Ching C. Lau are Co-Corresponding authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Teo, WY., Elghetany, M.T., Shen, J. et al. Therapeutic implications of CD1d expression and tumor-infiltrating macrophages in pediatric medulloblastomas. J Neurooncol 120, 293–301 (2014). https://doi.org/10.1007/s11060-014-1572-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1572-5