Abstract
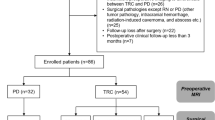
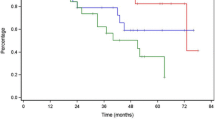
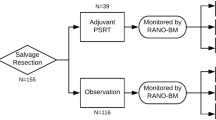
Brain metastases treated with stereotactic radiosurgery may show delayed enlargement on post-treatment imaging that is of ambiguous etiology. Histopathologic interpretation of brain specimens is often challenging due to the presence of significant radiation effects admixed with irradiated residual tumor of indeterminate viability. The purpose of this study was to assess the impact of histologic findings on clinical outcomes following resection of these lesions. Between 2004 and 2010, 690 patients with brain metastases were enrolled in a prospective gamma knife data repository, and lesions requiring excision were identified. Tissue specimens were divided into four groups based on the ratio of treatment related inflammatory changes (TRIC) to tumor cells, and subsequently patient outcomes were assessed. Of 2,583 metastases treated, 36 were excised due to symptomatic enlargement. Only TRIC, without residual evidence of tumor, was seen in 36 % (13/36) of specimens. Resection of these lesions resulted in 100 % local control in follow-up. Of the remaining 23 lesions that contained any viable-appearing tumor within the resected specimen, 8 recurred after resection. Lesions that enlarged in the first 6 months were more likely to contain higher amounts of residual tumor cells. Patients with even <2 % tumors cells on excision had significantly worse local control (75 vs. 100 %, p = 0.024) and survival (HR 0.27, p = 0.029) compared with those patients with exclusively TRIC. In summary, our findings underscore the importance of surgically obtaining tissue in a method that facilitates complete lesional interpretive histology in order to accurately guide ongoing patient management.




Similar content being viewed by others
References
Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R (1997) Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 37:745–751
Lunsford LD, Flickinger J, Lindner G, Maitz A (1989) Stereotactic radiosurgery of the brain using the first United States 201 cobalt-60 source gamma knife. Neurosurgery 24:151–159
Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary JP, Souhami L, Rotman M, Mehta MP, Curran WJ Jr (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 363:1665–1672. doi:10.1016/S0140-6736(04)16250-8
Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC (1999) Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys 45:427–434
Sneed PK, Suh JH, Goetsch SJ, Sanghavi SN, Chappell R, Buatti JM, Regine WF, Weltman E, King VJ, Breneman JC, Sperduto PW, Mehta MP (2002) A multi-institutional review of radiosurgery alone versus radiosurgery with whole brain radiotherapy as the initial management of brain metastases. Int J Radiat Oncol Biol Phys 53:519–526
Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, Kunieda E, Inomata T, Hayakawa K, Katoh N, Kobashi G (2006) Stereotactic radiosurgery plus whole-brain radiation therapy versus stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. J Am Med Assoc 295:2483–2491. doi:10.1001/jama.295.21.2483
Kocher M, Soffietti R, Abacioglu U, Villa S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD, Carrie C, Ben Hassel M, Kouri M, Valeinis E, van den Berge D, Collette S, Collette L, Mueller RP (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol 29:134–141. doi:10.1200/JCO.2010.30.1655
Patel TR, McHugh BJ, Bi WL, Minja FJ, Knisely JP, Chiang VL (2011) A comprehensive review of MR imaging changes following radiosurgery to 500 brain metastases. Am J Neuroradiol 32:1885–1892. doi:10.3174/ajnr.A2668
Sheehan JP, Sun MH, Kondziolka D, Flickinger J, Lunsford LD (2002) Radiosurgery for non-small cell lung carcinoma metastatic to the brain: long-term outcomes and prognostic factors influencing patient survival time and local tumor control. J Neurosurg 97:1276–1281. doi:10.3171/jns.2002.97.6.1276
Sheehan JP, Sun MH, Kondziolka D, Flickinger J, Lunsford LD (2003) Radiosurgery in patients with renal cell carcinoma metastasis to the brain: long-term outcomes and prognostic factors influencing survival and local tumor control. J Neurosurg 98:342–349. doi:10.3171/jns.2003.98.2.0342
Park HS, Yu JB, Knisely JPS, Chiang VL (2011) Outcomes following gamma knife for metastases. In: Mathieu D (ed) Gamma knife radiosurgery in tech. Rijeka, Croatia, pp 3–28
Szeifert GT, Kondziolka D, Levivier M, Lunsford LD (2012) Histopathology of brain metastases after radiosurgery. Prog Neurol Surg 25:30–38. doi:10.1159/000331169
Rauch PJ, Park HS, Knisely JP, Chiang VL, Vortmeyer AO (2012) Delayed radiation-induced vasculitic leukoencephalopathy. Int J Radiat Oncol Biol Phys 83:369–375. doi:10.1016/j.ijrobp.2011.06.1982
Jain R, Narang J, Sundgren PM, Hearshen D, Saksena S, Rock JP, Gutierrez J, Mikkelsen T (2010) Treatment induced necrosis versus recurrent/progressing brain tumor: going beyond the boundaries of conventional morphologic imaging. J Neurooncol 100:17–29. doi:10.1007/s11060-010-0139-3
Kano H, Kondziolka D, Lobato-Polo J, Zorro O, Flickinger JC, Lunsford LD (2010) T1/T2 matching to differentiate tumor growth from radiation effects after stereotactic radiosurgery. Neurosurgery 66:486–491. doi:10.1227/01.NEU.0000360391.35749.A5 Discussion 491–482
Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, Farnan N (2000) Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys 47:291–298
Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, Bhatt A, Jensen AW, Brown PD, Shih HA, Kirkpatrick J, Gaspar LE, Fiveash JB, Chiang V, Knisely JP, Sperduto CM, Lin N, Mehta M (2012) Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 30:419–425. doi:10.1200/JCO.2011.38.0527
Kondziolka D, Martin JJ, Flickinger JC, Friedland DM, Brufsky AM, Baar J, Agarwala S, Kirkwood JM, Lunsford LD (2005) Long-term survivors after gamma knife radiosurgery for brain metastases. Cancer 104:2784–2791. doi:10.1002/cncr.21545
Szeifert GT, Kondziolka D, Atteberry DS, Salmon I, Rorive S, Levivier M, Lunsford LD (2007) Radiosurgical pathology of brain tumors: metastases, schwannomas, meningiomas, astrocytomas, hemangioblastomas. Prog Neurolog Surg 20:91–105. doi:10.1159/0000100098
Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, Prabhu S, Loghin M, Gilbert MR, Jackson EF (2011) Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys 79:1487–1495. doi:10.1016/j.ijrobp.2009.12.061
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nath, S.K., Sheridan, A.D., Rauch, P.J. et al. Significance of histology in determining management of lesions regrowing after radiosurgery. J Neurooncol 117, 303–310 (2014). https://doi.org/10.1007/s11060-014-1389-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1389-2