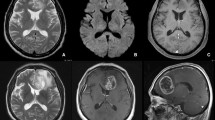
Abstract
Hemorrhage is common in brain tumors. Due to characteristic magnetic field changes induced by hemosiderin it can be detected using susceptibility weighted MRI (SWI). Its relevance to clinical syndromes is unclear. Here we investigated the patterns of intra-tumoral SWI positivity (SWIpos) as a surrogate for hemosiderin with regard to the prevalence of epilepsy. We report on 105 patients with newly diagnosed supra-tentorial gliomas and brain metastasis. The following parameters were recorded from pre-operative MRI: (1) SWIpos defined as dot-like or fine linear signal changes; (2) allocation of SWIpos to tumor compartments (contrast enhancement, central hypointensity, non-enhancing area outside contrast-enhancement); (3) allocation of SWIpos to include the cortex, or SWIpos in subcortical tumor parts only; (4) tumor size on T2 weighted and gadolinium-enhanced T1 images. 80 tumors (76 %) showed SWIpos (4/14 diffuse astrocytoma WHO II, 5/9 anaplastic astrocytoma WHO III, 41/46 glioblastoma WHO IV, 30/36 metastasis). The presence of SWIpos depended on tumor size but not on patient’s age, medication with antiplatelet drugs or anticoagulation. Seizures occurred in 60 % of patients. Cortical SWIpos significantly correlated with seizures in brain metastasis (p = 0.044), and as a trend in glioblastoma (p = 0.062). Cortical SWIpos may confer a risk for seizures in patients with newly diagnosed brain metastasis and glioblastoma. Whether development of cortical SWIpos induced by treatment or by the natural course of tumors also leads to the new onset of seizures has to be addressed in longitudinal studies in larger patient cohorts.


Similar content being viewed by others
References
Kondziolka D, Bernstein M, Resch L, Tator CH, Fleming JF, Vanderlinden RG, Schutz H (1987) Significance of hemorrhage into brain tumors: clinicopathological study. J Neurosurg 67:852–857
Rosen AD, Frumin NV (1979) Focal epileptogenesis after intracortical hemoglobin injection. Exp Neurol 66:277–284
Ueda Y, Willmore LJ, Triggs WJ (1998) Amygdalar injection of FeCl3 causes spontaneous recurrent seizures. Exp Neurol 153:123–127
Moran NF, Fish DR, Kitchen N, Shorvon S, Kendall BE, Stevens JM (1999) Supratentorial cavernous haemangiomas and epilepsy: a review of the literature and case series. J Neurol Neurosurg Psychiatry 66:561–568
van Breemen MS, Wilms EB, Vecht CJ (2007) Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol 6:421–430
Beaumont A, Whittle IR (2000) The pathogenesis of tumour associated epilepsy. Acta Neurochir (Wien) 142:1–15
Robinson RJ, Bhuta S (2011) Susceptibility-weighted imaging of the brain: current utility and potential applications. J Neuroimaging 21:e189–e204
Park MJ, Kim HS, Jahng GH, Ryu CW, Park SM, Kim SY (2009) Semiquantitative assessment of intra-tumoral susceptibility signals using non-contrast-enhanced high-field high-resolution susceptibility-weighted imaging in patients with gliomas: comparison with MR perfusion imaging. AJNR Am J Neuroradiol 30:1402–1408
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Li C, Ai B, Li Y, Qi H, Wu L (2010) Susceptibility-weighted imaging in grading brain astrocytomas. Eur J Radiol 75:e81–e85
Liwnicz BH, Wu SZ, Tew JM Jr (1987) The relationship between the capillary structure and hemorrhage in gliomas. J Neurosurg 66:536–541
Kothbauer P, Jellinger K, Falment H (1979) Primary brain tumour presenting as spontaneous intracerebral haemorrhage. Acta Neurochir (Wien) 49:35–45
Bradley WG Jr (1993) MR appearance of hemorrhage in the brain. Radiology 189:15–26
Allkemper T, Tombach B, Schwindt W, Kugel H, Schilling M, Debus O, Möllmann F, Heindel W (2004) Acute and subacute intracerebral hemorrhages: comparison of MR imaging at 1.5 and 3.0 T—initial experience. Radiology 232:874–881
Werring DJ, Frazer DW, Coward LJ, Losseff NA, Watt H, Cipolotti L, Brown MM, Jäger HR (2004) Cognitive dysfunction in patients with cerebral microbleeds on T2*-weighted gradient-echo MRI. Brain 127:2265–2275
Kamida T, Takeda Y, Fujiki M, Abe T, Abe E, Kobayashi H (2007) Nitric oxide synthase and NMDA receptor expressions in cavernoma tissues with epileptogenesis. Acta Neurol Scand 116:368–373
Friedman A, Kaufer D, Heinemann U (2009) Blood–brain barrier breakdown-inducing astrocytic transformation: novel targets for the prevention of epilepsy. Epilepsy Res 85:142–149
You G, Sha Z-Y, Yan W et al (2012) Seizure characteristics and outcomes in 508 Chinese adult patients undergoing primary resection of low-grade gliomas: a clinicopathological study. Neuro-Oncology 14:230–241
Berberat J, Grobholz R, Boxheimer L, Remonda L, Roelcke U (2012) Differentiating calcification and hemorrhage in tumor tissue using susceptibility weighted imaging [abstract]. Proc Int Soc Magn Res Med 20:3197
Noyce AJ, McCrae S, Gawler J, Evanson J (2010) Teaching neuroImages: microhemorrhages resulting from cranial radiotherapy in childhood. Neurology 75:e2–e3
Zeng QS, Kang XS, Li CF, Zhou GY (2011) Detection of hemorrhagic hypointense foci in radiation injury region using susceptibility-weighted imaging. Acta Radiol 52:115–119
Goldlust SA, Hsu M, Lassman AB, Panageas KS, Avila EK (2012) Seizure prophylaxis and melanoma brain metastases. J Neurooncol 108:109–114
Acknowledgments
The authors thank Professor Rainer Grobholz, Dept. Pathology, Cantonal Hospital Aarau, for sharing his expertise and discussion on intra-tumoral hemorrhage and manuscript reading.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
The study complies with the current laws and was approved by the Ethics Committee of the Cantonal Hospital, Aarau, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roelcke, U., Boxheimer, L., Fathi, A.R. et al. Cortical hemosiderin is associated with seizures in patients with newly diagnosed malignant brain tumors. J Neurooncol 115, 463–468 (2013). https://doi.org/10.1007/s11060-013-1247-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-013-1247-7