Abstract
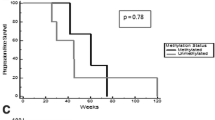
Verubulin (MPC-6827) is a microtubule-destabilizing agent that achieves high concentrations in the brain. Verubulin disrupts newly formed blood vessels in xenografts. We determined the safety and tolerability of verubulin administered in combination with carboplatin in patients with relapsed glioblastoma multiforme (GBM). Three pre-selected doses of verubulin were tested: 2.1, 2.7, and 3.3 mg/m2 in a standard “3+3” design. Verubulin was given every second week of a 6-week cycle in the 2.1 mg/m2 cohort or weekly for 3 weeks of a 4-week cycle in subsequent cohorts. Carboplatin was administered intravenously at an area under the curve (AUC) dosage 4 every 2 weeks for the 2.1 mg/m2 cohort or on day 1 of each 4-week cycle in subsequent cohorts. Nineteen patients with GBM in first or second relapse were enrolled. Four patients (21 %) experienced a grade 3 or greater verubulin- or carboplatin-related adverse event, including hypesthesia, cerebral ischemia, anemia, and thrombocytopenia. The mean plasma half life of verubulin was 3.2 h (SD = 0.82). Two patients achieved at least a partial response by Macdonald criteria. One of these patients remains progression free and off treatment more than 24 months beyond his initiation of verubulin. Five patients had stable disease. Median progression-free survival (PFS) across all patients was 8 weeks, and the 6-month PFS rate was 21 %. The combination of verubulin at the previously determined single-agent maximum tolerated dose of 3.3 mg/m2 with carboplatin in patients with recurrent/refractory GBM is safe and well tolerated. In this patient population with a highly vascularized tumor, no cerebral hemorrhage was observed.

Similar content being viewed by others
References
Central Brain Tumor Registry of the United States (CBTRUS) (2011) 2011 CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2004–2007. www.cbtrus.org/reports/reports.html. Accessed October 27, 2011
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomized phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Lamborn KR, Yung WK, Chang SM, Wen PY, Cloughesy TF, DeAngelis LM, Robins HI, Lieberman FS, Fine HA, Fink KL, Junck L, Abrey L, Gilbert MR, Mehta M, Kuhn JG, Aldape KD, Hibberts J, Peterson PM, Prados MD (2008) Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol 10:162–170
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740
Kasibhatla S, Baichwal V, Cai SX, Roth B, Skvortsova I, Skvortsov S, Lukas P, English NM, Sirisoma N, Drewe J, Pervin A, Tseng B, Carlson RO, Pleiman CM (2007) MPC-6827: a small-molecule inhibitor of microtubule formation that is not a substrate for multidrug resistance pumps. Cancer Res 67:5865–5871
Chang SM, Kuhn JG, Robins HI, Schold SC Jr, Spence AM, Berger MS, Mehta M, Pollack IF, Rankin C, Prados MD (2001) A phase II study of paclitaxel in patients with recurrent malignant glioma using different doses depending upon the concomitant use of anticonvulsants: a North American Brain Tumor Consortium report. Cancer 91:417–422
Pleiman C, Valppu L, Bhoite L, Austin H, Taylor J, Baichwal V, Laughlin M, Carlson R (2007) Vascular disruption effects of MPC-6827. 98th AACR Annual Meeting, Los Angeles, CA
Jessing K, Mauck K, Bradford C, Patton JS, Reeves L, Bulka K, Zhang H, Sirosoma N, Cai SX, Mather G (2005) MPC-6827, a small molecule inhibitor of microtubule formation with high brain penetration: absorption, distribution, metabolism, excretion, and clinical considerations. AACR Annual Meeting. AACR, Anaheim, CA, p 801-b
Tsimberidou AM, Akerley W, Schabel MC, Hong DS, Uehara C, Chhabra A, Warren T, Mather GG, Evans BA, Woodland DP, Swabb EA, Kurzrock R (2010) Phase I clinical trial of MPC-6827 (Azixa), a microtubule destabilizing agent, in patients with advanced cancer. Mol Cancer Ther 9:3410–3419
Warnick RE, Prados MD, Mack EE, Chandler KL, Doz F, Rabbitt JE, Malec MK (1994) A phase II study of intravenous carboplatin for the treatment of recurrent gliomas. J Neurooncol 19:69–74
van Warmerdam LJ, Rodenhuis S, ten Bokkel Huinink WW, Maes RA, Beijnen JH (1995) The use of the Calvert formula to determine the optimal carboplatin dosage. J Cancer Res Clin Oncol 121:478–486
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
de Groot JF, Gilbert MR, Aldape K, Hess KR, Hanna TA, Ictech S, Groves MD, Conrad C, Colman H, Puduvalli VK, Levin V, Yung WK (2008) Phase II study of carboplatin and erlotinib (Tarceva, OSI-774) in patients with recurrent glioblastoma. J Neurooncol 90:89–97
Acknowledgments
We thank Kristin Kraus, MS, for editorial assistance in preparing this paper. This project was funded by Myrexis Inc. (formerly Myriad Pharmaceuticals).
Conflict of interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Kenneth F. Grossmann and Howard Colman contributed equally to this study.
Rights and permissions
About this article
Cite this article
Grossmann, K.F., Colman, H., Akerley, W.A. et al. Phase I trial of verubulin (MPC-6827) plus carboplatin in patients with relapsed glioblastoma multiforme. J Neurooncol 110, 257–264 (2012). https://doi.org/10.1007/s11060-012-0964-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-012-0964-7