Abstract
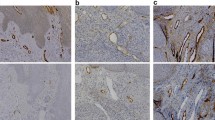
Increased lymphatic density correlates with lymph node metastases and survival in some epithelial cancers. The transcription factor, Prospero-related homeobox-gene 1, PROX1, plays an important role in the differentiation and proliferation of the lymphatic and nervous systems. We studied the clinicopathological significance of PROX1 expression in neuroblastomas (NBs) as the majority of patients have lymphatic and/or haematogenous metastases at diagnosis. PROX1-immunostained lymphatic vessels were present in 40/69 (58%) of NBs and 1/6 benign ganglioneuromas (GNs). Lymphatic density (LD) counts were significantly increased in NBs from patients with unfavourable clinical and pathological factors, and with distant lymph node metastases (LNM). Lymphatic invasion (LI) by tumoral emboli was present in 27/40 (68%) of NBs. A significantly higher proportion of LI was seen in undifferentiated/poorly-differentiated, (UD/PD) compared with differentiated NBs. LI was increased in NBs from patients with advanced-stage and high-risk group. Nuclear-PROX1 expression in tumoral cells was present in 35/69 (51%) NBs but was absent in all GNs. PROX1 expression was significantly higher in UD/PD compared with differentiated NBs. It was also higher in NBs with all adverse clinicopathological and biological variables. LI, PROX1 cellular expression and high LD correlated with a shorter overall survival and event-free survival (EFS). Multivariable Cox regression analysis showed that the effect of LD on both OS and EFS was independent of mitosis-karyorrhexis index and MYCN amplification. Increased LD, LI and cellular expression correlated with adverse factors in NBs. Increased LD correlated with LNM suggesting that PROX1 contributes to neuroblastoma progression and lymphatic spread.


Similar content being viewed by others
References
Maris JM (2010) Recent advances in neuroblastoma. N Engl J Med 362:2202–2211
Cotterill SJ, Pearson AD, Pritchard J, Foot AB, Roald B, Kohler JA, Imeson J (2000) Clinical prognostic factors in 1277 patients with neuroblastoma: results of The European Neuroblastoma Study Group ‘Survey’ 1982–1992. Eur J Cancer 36:901–908
Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM, Faldum A, Hero B, Iehara T, Machin D, Mosseri V, Simon T, Garaventa A, Castel V, Matthay KK (2009) The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol 27:289–297
Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B, Stram DO, Gerbing RB, Lukens JN, Matthay KK, Castleberry RP (1999) The international neuroblastoma pathology classification (the Shimada system). Cancer 86:364–372
Rossler J, Taylor M, Geoerger B, Farace F, Lagodny J, Peschka-Suss R, Niemeyer CM, Vassal G (2008) Angiogenesis as a target in neuroblastoma. Eur J Cancer 44:1645–1656
Lagodny J, Juttner E, Kayser G, Niemeyer CM, Rossler J (2007) Lymphangiogenesis and its regulation in human neuroblastoma. Biochem Biophys Res Commun 352:571–577
Ribatti D, Nico B, Cimpean AM, Raica M (2010) Podoplanin and LYVE-1 expression in lymphatic vessels of human neuroblastoma. J Neurooncol 100:151–152
Becker J, Wang B, Pavlakovic H, Buttler K, Wilting J (2010) Homeobox transcription factor Prox1 in sympathetic ganglia of vertebrate embryos: correlation with human stage 4s neuroblastoma. Pediatr Res 68:112–117
Becker J, Pavlakovic H, Ludewig F, Wilting F, Weich HA, Albuquerque R, Ambati J, Wilting J (2010) Neuroblastoma progression correlates with downregulation of the lymphangiogenesis inhibitors VEGFR-2. Clin Cancer Res 16:1431–1441
Eggert A, Ikegaki N, Kwiatkowski J, Zhao H, Brodeur GM, Himelstein BP (2000) High-level expression of angiogenic factors is associated with advanced tumor stage in human neuroblastomas. Clin Cancer Res 6:1900–1908
Komuro H, Kaneko S, Kaneko M, Nakanishi Y (2001) Expression of angiogenic factors and tumor progression in human neuroblastoma. J Cancer Res Clin Oncol 127:739–743
Nowicki M, Konwerska A, Ostalska-Nowicka D, Derwich K, Miskowiak B, Kondraciuk B, Samulak D, Witt M (2008) Vascular endothelial growth factor (VEGF)-C: a potent risk factor in children diagnosed with stadium 4 neuroblastoma. Folia Histochem Cytobiol 46:493–499
Sleeman JP, Thiele W (2009) Tumor metastasis and the lymphatic vasculature. Int J Cancer 125:2747–2756
Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G (2002) An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J 21:1505–1513
Griffiths RL, Hidalgo A (2004) Prospero maintains the mitotic potential of glial precursors enabling them to respond to neurons. EMBO J 23:2440–2450
Van der Auwera I, Cao Y, Tille JC, Pepper MS, Jackson DG, Fox SB, Harris AL, Dirix LY, Vermeulen PB (2006) First international consensus on the methodology of lymphangiogenesis quantification in solid human tumours. Br J Cancer 95:1611–1625
Dungwa J, Uparkar U, May M, Ramani P (2012) Angiogenin up-regulation correlates with adverse clinicopathological and biological factors, increased microvascular density and poor patient outcome in neuroblastomas. Histopathology. doi:10:1111/j.1365-2559.2012.04176.x
Hong YK, Detmar M (2003) Prox1, master regulator of the lymphatic vasculature phenotype. Cell Tissue Res 314:85–92
Dadras SS, Skrzypek A, Nguyen L, Shin JW, Schulz MM, Arbiser J, Mihm MC, Detmar M (2008) Prox-1 promotes invasion of kaposiform hemangioendotheliomas. J Invest Dermatol 128:2798–2806
Sleeman J, Schmid A, Thiele W (2009) Tumor lymphatics. Semin Cancer Biol 19:285–297
Scotting PJ, Walker DA, Perilongo G (2005) Childhood solid tumours: a developmental disorder. Nat Rev Cancer 5:481–488
Torii M, Matsuzaki F, Osumi N, Kaibuchi K, Nakamura S, Casarosa S, Guillemot F, Nakafuku M (1999) Transcription factors Mash-1 and Prox-1 delineate early steps in differentiation of neural stem cells in the developing central nervous system. Development 126:443–456
Elsir T, Eriksson A, Orrego A, Lindstrom MS, Nister M (2010) Expression of PROX1 is a common feature of high-grade malignant astrocytic gliomas. J Neuropathol Exp Neurol 69:129–138
Petrova TV, Nykanen A, Norrmen C, Ivanov KI, Andersson LC, Haglund C, Puolakkainen P, Wempe F, von Melchner H, Gradwohl G, Vanharanta S, Aaltonen LA, Saharinen J, Gentile M, Clarke A, Taipale J, Oliver G, Alitalo K (2008) Transcription factor PROX1 induces colon cancer progression by promoting the transition from benign to highly dysplastic phenotype. Cancer Cell 13:407–419
Shimoda M, Takahashi M, Yoshimoto T, Kono T, Ikai I, Kubo H (2006) A homeobox protein, prox1, is involved in the differentiation, proliferation, and prognosis in hepatocellular carcinoma. Clin Cancer Res 12:6005–6011
Versmold B, Felsberg J, Mikeska T, Ehrentraut D, Kohler J, Hampl JA, Rohn G, Niederacher D, Betz B, Hellmich M, Pietsch T, Schmutzler RK, Waha A (2007) Epigenetic silencing of the candidate tumor suppressor gene PROX1 in sporadic breast cancer. Int J Cancer 121:547–554
Yoshimoto T, Takahashi M, Nagayama S, Watanabe G, Shimada Y, Sakasi Y, Kubo H (2007) RNA mutations of prox1 detected in human esophageal cancer cells by the shifted termination assay. Biochem Biophys Res Commun 359:258–262
Acknowledgments
The authors thank Above and Beyond Charities of the University Hospitals Bristol Foundation Trust and Ince and Company for their financial support. The authors are grateful to Mr. P.B. Savage for providing laboratory facilities, and to Josiah Dungwa, Alison Headford, Emile Sowa Avugrah and Mike Luckett for technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramani, P., Norton, A., Somerville, M.S. et al. PROX1 lymphatic density correlates with adverse clinicopathological factors, lymph node metastases and survival in neuroblastomas. J Neurooncol 108, 375–383 (2012). https://doi.org/10.1007/s11060-012-0838-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-012-0838-z