Abstract
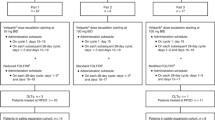
Irinotecan has radiosensitizing effects and shows synergism with nitrosoureas. We performed a Phase II study of RT and irinotecan, followed by BCNU plus irinotecan in newly-diagnosed GBM. The MTD for patients receiving enzyme-inducing anticonvulsants (EIAC) was as follows: irinotecan 400 mg/m2/week on Days 1, 8, 22 and 29 during RT, followed by BCNU 100 mg/m2 Day 1, and irinotecan, 400 mg/m2 on Days 1, 8, 22 and 29, every 6 weeks. The MTD for non-EIAC patients was as follows: irinotecan 125 mg/m2/week on Days 1, 8, 22 and 29 during RT, followed by BCNU 100 mg/m2 Day 1 and irinotecan 75 mg/m2 Days 1, 8, 22 and 29, every 6 weeks. Median OS was 10.8 mos. (95% CI: 7.7–14.9); OS at 12 months was 44.6% (95% CI: 33.3–59.8) and PFS 6 was 28.6% (95% CI: 18.9–43.2). Patients went off treatment due to adverse events (7%), refusal (11%), progressive disease (48%), death (9%), and other (9%); 16% completed protocol treatment. Survival was similar in patients with variant (6/7 or 7/7) and wild-type (6/6) UGT1A1*28 genotypic alleles. Grade 3–4 toxicity was more common in non-EIAC patients with variant alleles. SN-38 Cmax and AUC in EIAC patients receiving 400 mg/m2 irinotecan were 20.9 ng/ml and 212 ng/ml h, and in non-EIAC patients receiving 125 mg/m2, 15.5 ng/ml and 207 ng/ml h. SN-38 AUC varied by UGT1A1*28 status in non-EIAC patients. This regimen was not significantly active and radiosensitization was not observed. Non-EIAC patients with UGT1A1*28 variant alleles appear particularly sensitive to toxicity from irinotecan.



Similar content being viewed by others
References
Tamura K, Takada M, Kawase I et al (1997) Enhancement of tumor radio-response by irinotecan in human lung tumor xenografts. Jpn J Cancer Res 88:218–223
Coggins CA, Elion GB, Houghton PJ et al (1998) Enhancement of irinotecan activity against central nervous system tumor xenografts by alkylating agents. Cancer Chemother Pharmacol 41:485–490
Castellino RC, Elion GB, Keir ST et al (2000) Schedule-dependent activity of irinotecan plus BCNU against human malignant glioma xenografts. Cancer Chemother Pharmacol 45:345–349
Sasai K, Guo GZ, Shibuya K et al (1998) Effects of SN-38 (an active metabolite of irinotecan) on responses of human and rodent cells to irradiation. Int J Radiat Oncol Biol Phys 42:785–788
Omura M, Torigoe S, Kubota N (1997) SN-38, a metabolite of the camptothecin derivative irinotecan, potentiates of cytotoxic effect of radiation in human colon adenocarcinoma cells grown as spheroids. Radiother Oncol 43:197–201
Kaplan E, Meier P (1958) Nonparametric estimation from incomplete observation. J Am Stat Assoc 53:457–481
Galanis E, Buckner JC, Maurer MJ et al (2005) Phase II trial of temsirolomus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol 23:5294–5304
Santisteban M, Buckner JC, Reid JM et al (2009) Phase II trial of two different irinotecan schedules with pharmacokinetic analysis in patients with recurrent glioma: North Central Cancer Treatment Group results. J Neurooncol 92:165–175
Buckner JC, Reid JM, Wright K et al (2003) Irinotecan in the treatment of glioma patients: current and future studies of the North Central Cancer Treatment Group. Cancer 97(Suppl):2352–2358
Ando Y, Saka H, Asai G et al (1998) UGT1A1 genotypes and glucuronidation of SN-38, the active metabolite of irinotecan. Ann Oncol 9:845–847
Iyer L, Hall D, Mortell MA, Ramirez J et al (1998) Phenotype-genotype correlation of in vitro SN-38 (active metabolite of irinotecan) and bilirubin glucuronidation in human liver tissue with UGT1A1 promoter polymorphism. Clin Pharmacol Ther 65:576–582
Reid JM, Buckner JC, Schaaf LF et al (2000) Anticonvulsants alter the pharmacokinetics of irinotecan in patients with recurrent glioma. Proc Am Soc Clin Oncol 19:160a (Abstr)
Gajjar AJ, Radomski KM, Bowers DC et al. (2000) Pharmacokinetics of irinotecan and metabolites in pediatric high-grade glioma patients with and without co-administration of enzyme-inducing anticonvulsants. Proc Am Soc Clin Oncol 19(Suppl):162a (Abstr)
Odani A, Hashimoto Y, Otsuki Y et al (1997) Genetic polymorphism of the CYP2C subfamily and its effect on the pharmacokinetics of phenytoin in Japanese patients with epilepsy. Clin Pharmacol Ther 62:287–292
Slatter JG, Su P, Sams JP et al (1997) Bioactivation of the anticancer agent irinotecan to SN-38 by human hepatic microsomal carboxylesterases and the in vitro assessment of potential drug interactions. Drug Metab Dispos 25:1157–1164
Gilbert M, Supko J, Batchelor T et al (2003) Phase I clinical and pharmacokinetic study of irinotecan in adults with recurrent malignant glioma. Clin Cancer Res 9:2940–2949
Kuhn JG (2002) Influence of anticonvulsants on the metabolism and elimination of irinotecan. A North American Brain Tumor Consortium preliminary report. Oncology 16(suppl):33–40
Cloughesy TF, Filka E, Nelson G (2002) Irinotecan treatment for recurrent malignant glioma using an every 3-week regimen. Am J Clin Oncol 25:204–208
Cloughesy TF, Filka E, Kuhn J (2003) Two studies evaluating irinotecan treatment for recurrent malignant glioma using an every-3-week regimen. Cancer 97:2381–2386
Friedman HS, Petros WP, Friedman AH et al (1999) Irinotecan therapy in adults with recurrent or progressive malignant glioma. J Clin Oncol 17:1516–1525
Chamberlain MD (2002) Salvage chemotherapy with irinotecan for recurrent glioblastoma multiforme. J Neurooncol 56:183–188
Fukuda M, Soda H, Fukuda M et al (2007) Irinotecan and cisplatin with concurrent split-course radiotherapy in locally advanced non small-cell lung cancer: a multi-institutional phase 2 study. Cancer 110:606–613
Raymond E, Fabbro M, Goige V et al (2003) Multicentre phase II study and pharmacokinetic analysis of irinotecan in chemotherapy—naïve patients with glioblastoma. Ann Oncol 14:604–614
Fountzilas G, Karkavelas G, Kalogera-Fountzila A et al (2006) Post-operative combined radiation and chemotherapy with temozolomide and irinotecan in patients with high-grade astrocytic tumors. A phase II study with biomarker evaluation. Anticancer Res 26:4675–4686
Stewart CF, Zamboni WC, Crom WR et al (1997) Disposition of irinotecan and SN-38 following oral and intravenous irinotecan dosing in mice. Cancer Chemother Pharmacol 40:259–265
Hoskins JM, Goldberg RM, Qu P et al (2007) UGT1A1*28 genotype and irinotecan-induced neutropenia: dose matters. J Natl Cancer Inst 99:1290–1295
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Green SB, Byar DP, Walker MD et al (1983) Comparisons of carmustine, procarbazine, and high-dose methylprednisolone as additions to surgery and radiotherapy for the treatment of malignant glioma. Cancer Treat Rep 67:121–132
Fine HA, Dear KB, Loeffler JS (1993) Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer 71:2585–2597
Shaw EG, Wang M, Coons S et al (2008) Final report of Radiation Therapy Oncology Group (RTOG) protocol 9802: radiation therapy (RT) versus RT + procarbazine, CCNU and vincristine (PCV) chemotherapy for adult low-grade glioma (LGG). J Clin Oncol 26(15S):90s (Abstr)
Lassman AB, Panageas KS, Iwamoto FM et al (2009) International retrospective study of 1001 adults with anaplastic oligodendroglial tumors. Neurooncol 11:629 (Abstr)
Wolfgang WW, Weller M (2008) NOA-4 randomized phase IIII study of sequential radiochemothearpy of anaplastic glioma with PCV or temozolomide. J Clin Oncol 26(18S, Part II):1008s (Abstr)
Friedman HS, Prados MD, Wen PY et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-35269, CA-35101, CA-35103, CA-35113, CA-35431, CA-35267, CA-52352, CA-37417, CA-63848, and by grants from Pharmacia and Upjohn.
The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute or the National Institute of Health.
Additional participating institutions include in Appendix.
Appendix
Appendix
Additional participating institutions include: Rapid City Regional Oncology Group, Rapid City, SD 59709 (Richard Tenglin, M.D.); Mayo Clinic Arizona, Scottsdale, AZ 85259 (Tom R. Fitch, M.D.); CentraCare Clinic, St. Cloud, MN 56301 (Donald Jurgens, M.D.); Illinois Oncology Research Assn. CCOP, Peoria, IL 61602 (John W. Kugler, M.D.); Cedar Rapids Oncology Project CCOP, Cedar Rapids, IA 52403 (Martin Wiesenfeld, M.D.); Michigan Cancer Research Consortium, Ann Arbor, MI 48106 (Philip J. Stella, M.D.); Metro-Minnesota Community Clinical Oncology Program, St. Louis Park, MN 55416 (Patrick J. Flynn, M.D.); Sioux Community Cancer Consortium, Sioux Falls, SD 57105 (Loren K. Tschetter, M.D.); Wichita Community Clinical Oncology Program, Wichita, KS 67214-3882 (Shaker R. Dakhil, M.D.).
Rights and permissions
About this article
Cite this article
Jaeckle, K.A., Ballman, K.V., Giannini, C. et al. Phase II NCCTG trial of RT + irinotecan and adjuvant BCNU plus irinotecan for newly diagnosed GBM. J Neurooncol 99, 73–80 (2010). https://doi.org/10.1007/s11060-009-0103-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-009-0103-2