Abstract
Objective
Aminolevulinic acid (ALA)-mediated photodynamic therapy (PDT) may represent a treatment option for malignant brain tumors. We used a three-dimensional cell culture system, the C6 glioma spheroid model, to study acute effects of PDT and how they might be influenced by treatment conditions.
Methods
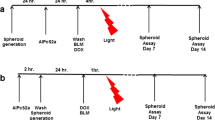
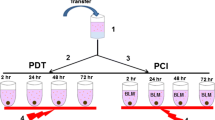
Spheroids were incubated for 4 h in 100 μg/ml ALA in 5% CO2 in room air or 95% O2 with subsequent irradiation using a diode laser (λ = 635 nm, 40 mW/cm2, total fluence 25 J/cm2). Control groups were “laser only”, “ALA only”, and “no drug no light”. Annexin V-FITC, a marker used for detection of apoptosis, propidium iodide (PI), a marker for necrotic cells and H 33342, a chromatin stain, were used for morphological characterization of PDT effects by confocal laser scanning and fluorescence microscopy. Hematoxylin–eosin staining and TdT-FragEL (TUNEL) assay were used on cryosections. Growth kinetics were followed for 8 days after PDT.
Results
PDT after incubation in 5% CO2 provided incomplete cell death and growth delay in spheroids of >350 μm diameter. However, complete cell death and growth arrest occurred in smaller spheroids (<350 μm). Incubation in 95% O2 with subsequent PDT resulted in complete cell death and growth arrest regardless of spheroid size. In incompletely damaged spheroids viable cells were restricted to spheroid centers. The rate of cell death in all control groups was negligible. Cell death was accompanied by annexin/PI costaining, but there was also evidence for annexin V-FITC staining without PI uptake.
Conclusions
PDT of experimental glioma results in rapid and significant cell death that could be verified as acute necrosis immediately after irradiation. This effect depended on O2 concentration and spheroid size.











Similar content being viewed by others
References
Bell HS, Whittle IR, Walker M, Leaver HA, Wharton SB (2001) The development of necrosis and apoptosis in glioma: experimental findings using spheroid culture systems. Neuropathol Appl Neurobiol 27:291–304
Betz AL, Iannotti F, Hoff JT (1989) Brain edema: a classification based on blood–brain barrier integrity. Cerebrovasc Brain Metab Rev 1:133–154
Bigelow CE,Mitra S, Knuechel R, Forster TH (2001) ALA- and ALA-Hexylester-induced protoporphyrin IX fluorescence and distribution in multicell tumor. Br J Cancer 85:727–734
Brandes AA (2003) State-of-the-art treatment of high-grade brain tumors. Semin Oncol 30(Suppl 19):4–9
Brock CS, Bower M (1997) Current perspectives in gliomas. Med Oncol 14:103–120
Cheung-Hoi Y, Wong HK, Yung HW, Lau LT (2001) Ischemia-induced apoptosis in primary cultures of astrocytes. Glia 35:121–130
Dubessy C, Merlin JM, Marchal C, Guillemin F (2000) Spheroids in radiobiology and photodynamic therapy. Crit Rev Oncol Hematol 36:179–192
Eleouet S, Rousset N, Carre J, Bourre L, Vonarx V, Lajat Y, Beijersbergen van Henegouwen GM, Patrice T (2000) In vitro fluorescence, toxicity and phototoxicity induced by delta-aminolevulinic acid (ALA) or ALA-esters. Photochem Photobiol 71:447–454
van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP (1998) Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 31:1–9
Foster TH, Hartley DF, Nichols MG, Hilf R (1993) Fluence rate effects in photodynamic therapy of multicell tumor spheroids. Cancer Res 53:1249–1254
Friesen SA, Hjortland GO, Madsen SJ, Hirschberg H, Engebraten O, Nesland JM, Peng Q (2002) 5-Aminolevulinic acid-based photodynamic detection and therapy of brain tumors (Review). Int J Oncol 21:577–582
Georgakoudi I, Nichols MG, Foster TH (1999) The mechanism of Photofrin photobleaching and its consequences for photodynamic dosimetry. Photochem Photobiol 65:135–144
Graham SH, Chen J (2001) Programmed cell death in cerebral ischemia. J Cereb Blood Flow Metab 21:99–109
Hebeda KM, Saarnak AE, Olivo M, Sterenborg HJ, Wolbers JG et al (1998) 5-aminolevulinic acid induced endogenous porphyrin fluorescence in 9L and C6 brain tumours and in the normal rat brain. Acta Neurochir 140:503–512
Henderson BW, Dougherty TJ (1992) How does photodynamic therapy work? Photochem Photobiol 55:777–779
Hirschberg H, Sun CH, Tromberg BJ, Yeh AT, Madsen SJ (2004) Enhanced cytotoxic effects of 5-aminolevulinic acid mediated photodynamic therapy by concurrent hyperthermia in glioma spheroids. J Neurooncol 70:289–299
Ito S, Rachinger W, Stepp H, Reulen HJ, Stummer W (2005) Oedema formation in experimental photo-irradiation therapy of brain tumours using 5-ALA. Acta Neurochir (Wien) 147:57–65
Knappe A, Beyer W, Riesenberg R, Schneede P, Sroka R, Unsold E, Valet G (1995) Computer-controlled laser irradiation unit for studies of light-induced processes in cell cultures. Biomed Tech (Berl) 40:272–275
Kostron H, Obwegeser A, Jakober R (1996) Photodynamic therapy in neurosurgery: a review. J Photochem Photobiol B, Biol 36:157–168
Leist M, Jaattela M (2001) Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol 2:289–298
Macecek J, Kolarova H, Psotova J, Bajgar R, Huf M, Nevrelova P, Tomeeka M, Mosinger J (2004) Assessment of cellular damage by comet assay after photodynamic therapy in vitro. Acta Medica (Hradec Kralove) 47:327–329
Madsen SJ, Sun CH, Tromberg BJ, Hirschberg H (2001) Development of a novel indwelling balloon applicator for optimizing light delivery in photodynamic therapy. Lasers Surg Med 29:406–412
Madsen SJ, Sun CH, Tromberg BJ, Wallace VP, Hirschberg H (2000) Photodynamic therapy of human glioma spheroids using 5-aminolevulinic acid. Photochem Photobiol 72:128–134
Madsen SJ, Sun CH, Tromberg BJ, Hirschberg H (2003) Repetitive 5-aminolevulinic acid-mediated photodynamic therapy on human glioma spheroids. J Neurooncol 62:243–250
Madsen SJ, Sun CH, Tromberg BJ, Yeh AT, Sanchez R, Hirschberg H (2002) Effects of combined photodynamic therapy and ionizing radiation on human glioma spheroids. Photochem Photobiol 76:411–416
Mahaley MS Jr, Mettlin C, Natarajan N, Laws ER Jr, Peace BB (1998) National survey of patterns of care for brain-tumor patients. J Neurosurg 71:826–836
Muller PJ, Wilson BC (1995) Photodynamic therapy for recurrent supratentorial gliomas. Semin Surg Oncol 11:346–543
Mueller-Klieser W, Bourrat B, Gabbert H, Sutherland RM (1985) Changes in O2 consumption of multicellular spheroids during development of necrosis. Adv Exp Med Biol 191:775–784
Nichols MG, Foster TH (1994) Oxygen diffusion and reaction-kinetics in the photodynamic therapy of multicell tumor spheroids. Phys Med Biol 39:2161–2181
Nirmala C, Rao JS, Ruifrok AC, Langford LA, Obeyesekere M (2001) Growth characteristics of glioblastoma spheroids. Int J Oncol 19:1109–1115
Oleinick NL, Morris RL, Belichenko T (2002) The role of apoptosis in response to photodynamic therapy: what, where, why, and how. Photochem Photobiol Sci 1:1–21
Olzowy B, Hundt CS, Stocker S, Bise K, Reulen HJ, Stummer W (2002) Photoirradiation therapy of experimental malignant glioma with 5-aminolevulinic acid. J Neurosurg 97:970–976
Origitano TC, Caron MJ, Reichman OH (1994) Photodynamic therapy for intracranial neoplasms. Literature review and institutional experience. Mol Chem Neuropathol 21:337–352
Plaetzer K, Kiesslich T, Krammer B, Hammerl P (2002) Characterization of the cell death modes and the associated changes in cellular energy supply in response to AlPcS4-PDT. Photochem Photobiol Sci 1:172–177
Popovic EA, Kaye AH, Hill JS (1996) Photodynamic therapy of brain tumors. J Clin Laser Med Surg 14:251–261
Schellenberger EA, Bogdanov A Jr, Petrovsky A, Ntziachristos V, Weissleder R, Josephson L (2003) Optical imaging of apoptosis as a biomarker of tumor response to chemotherapy. Neoplasia 5:187–192
Stummer W, Gotz C, Hassan A, Heimann A, Kempski O (1993) Kinetics of Photofrin II in perifocal brain edema. Neurosurgery 33:1075–1081
Stummer W, Hassan A, Kempski O et al (1996) Photodynamic therapy within edematous brain tissue: considerations on sensitizer dose and time point of laser irradiation. J Photochem Photobiol B, Biol 36:179–181
Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ (2000) Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg 93:1003–1013
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, ALA-glioma study group (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Onc 7(5):392–401
Stummer W, Stocker S, Novotny A, Heimann A, Sauer O, Kempski O, Plesnila N, Wietzorrek J, Reulen HJ (1998) In vitro and in vivo porphyrin accumulation by C6 glioma cells after exposure to 5-aminolevulinic acid. J Photochem Photobiol B, Biol 45:160–169
Stummer W, Stocker S, Wagner S, Stepp H, Fritsch C, Goetz C, Goetz AE, Kiefmann R, Reulen HJ (1998) Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery 42:518–525
Tsai JC, Hsiao YY, Teng LJ, Chen CT, Kao MC (1999) Comparative study on the ALA photodynamic effects of human glioma and meningioma cells. Lasers Surg Med 24:296–305
Uehlinger P, Zellweger M, Wagnieres G, Juillerat-Jeanneret L, van den Bergh H, Lange N (2000) 5-Aminolevulinic acid and its derivatives: physical chemical properties and protoporphyrin IX formation in cultured cells. J Photochem Photobiol B, Biol 54:72–80
Wallner KE, Galicich JH, Krol G, Arbit E, Malkin MG (1989) Patterns of failure following treatment for glioblastoma-multiforme and anaplastic astocytoma. Int J Rad Oncol Biol Phys 16:1405–1409
Webber J, Kessel D, Fromm D (1997) Side effects and photosensitization of human tissues after aminolevulinic acid. J Surg Res 68(1):31–37
Acknowledgments
This work was supported by Deutsche Krebshilfe e.V. Projekt-Nr. 70–2864. We gratefully acknowledge advice from Jorg Cristian Tonn, Prof., MD, Department of Neurosurgery, Herbert Stepp, Ph.D., Tobias Beck, Dipl. phys., Laser Research Laboratory, Ludwig-Maximilians University, Munich, Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zelenkov, P., Baumgartner, R., Bise, K. et al. Acute morphological sequelae of photodynamic therapy with 5-aminolevulinic acid in the C6 spheroid model. J Neurooncol 82, 49–60 (2007). https://doi.org/10.1007/s11060-006-9252-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-006-9252-8