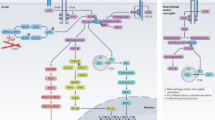
Studies have shown that tyrosine kinases (TK) may play an important role in the pathogenesis of multiple sclerosis (MS) operating through a microRNA regulatory network. MicroRNA network-based analysis revealed 17 receptor pathways activated by TK and regulated by miRNAs encoded at the DLK1-DIO3 locus on chromosome 14. TK are actively involved in the epigenetic regulation of the pathological process in MS involving the microRNA network, and have attracted attention as targets for the development of new directions in the treatment of MS. Bruton’s TK inhibitors (BTKI) have attracted the most attention, as they have demonstrated their ability to suppress the activity of autoimmune inflammatory lesions in a model of MS, i.e., experimental autoimmune encephalomyelitis (EAE). This is due to the influence of BTK on the activity of B cells, which play a critical role in the development of the pathological process. This review discusses the types of BTKI, the characteristics of their actions in EAE, and results from the first trials in MS.
Similar content being viewed by others
References
E. I. Gusev and A. N. Boyko, Multiple Sclerosis. Scientific and Applies Guidelines, ROOI Human Health (2020).
A. Ascherio and K. L. Munger, “Environmental risk factors for multiple sclerosis. Part I & II,” Ann. Neurol., 61, 288–299 and 504–513 (2007), https://doi.org/10.1002/ana.21117 and https://doi.org/10.1002/ana.21141.
N. A. Patsopoulos, S. E. Baranzini, A. Santaniello, et al., “Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility,” Science, 80, 365 (2019), https://doi.org/10.1126/science.aav7188.
H. A. Lawson, J. M. Cheverud, and J. B. Wolf, “Genomic imprinting and parent-of-origin effects on complex traits,” Nat. Rev. Genet., 14, 609–617 (2013), https://doi.org/10.1038/nrg3543.
A. N. Baulina, O. I. Kulakova, I. N. Kiselev, et al., “Immune-related miRNA expression patterns in peripheral blood mononuclear cells differ in multiple sclerosis relapse and remission,” J. Neuroimmunol., 317, 67–76 (2018), https://doi.org/10.1016/j.jneuroim.2018.01.005.
A. N. Baulina, G. A. Osmak, I. N. Kiselev, et al., “MiRNAs from DLK1-DIO3 Imprinted Locus at 14q32 are Associated with multiple sclerosis: Gender-specific expression and regulation of receptor tyrosine kinases signaling,” Cells, 8, No. 2, 133–140 (2019), https://doi.org/10.3390/cells8020133.
J. L. Huynh and P. Casaccia, “Epigenetic mechanisms in multiple sclerosis: implications for pathogenesis and treatment,” Lancet Neurol., 12, No. 2, 195–206 (2013), https://doi.org/10.1016/S1474-4422(12)70309-5.
G. Comi, A. Bar-Or, H. Lassmann, et al., “Role of B cells in multiple sclerosis and related disorders,” Ann. Neurol., 89, 13–23 (2021), https://doi.org/10.1002/ana.25927.
M. V. Melnikov, V. S. Rogovskii, et al., “Mechanisms of involvement of B lymphocytes in the pathogenesis of multiple sclerosis,” Med. Ekstrem. Situats., 2, 34–39 (2021).
S. Torke and M. S. Weber, “Inhibition of Bruton’s tyrosine kinase as a novel therapeutic approach in multiple sclerosis,” Expert Opin. Investig. Drugs, 29, No. 10, 1143–1150 (2020), https://doi.org/10.1080/13543784.2020.1807934.
Z. Pan, H. Scheerens, S. J. Li, et al., “Discovery of selective irreversible inhibitors for Bruton’s tyrosine kinase,” ChemMedChem, 2, 58–61 (2007), https://doi.org/10.1002/cmdc.200600221.
J. Zheng, J. Wu, X. Ding, et al., “Small molecule approaches to treat autoimmune and inflammatory diseases (Part I, kinase inhibitors,” Bioorg. Med. Chem. Lett., 38, 127862 (2021), https://doi.org/10.1016/j.bmcl.2021.127862.
R. W. Hendriks, S. Yuvaraj, and L. P. Kil, “Targeting Bruton’s tyrosine kinase in B cell malignancies,” Nat. Rev. Cancer, 14, 219–232 (2014), https://doi.org/10.1038/nrc3702.
C. Liang, D. Tian, X. Ren, et al., “The development of Bruton’s tyrosine kinase (BTK) inhibitors from 2012 to 2017: A mini-review,” Eur. J. Med. Chem., 151, 315–326 (2018), https://doi.org/10.1016/j.ejmech.2018.03.062.
H. Y. Estupiñán, A. Berglöf, R. Zain, and C. E. Smith, “Comparative analysis of BTK inhibitors and mechanisms underlying adverse effects,” Front. Cell. Dev. Biol., 9, 630942 (2021), https://doi.org/10.3389/fcell.2021.630942.
Q. Liu, Y. Sabnis, Z. Zhao, et al., “Developing irreversible inhibitors of the protein kinase cysteinome,” Chem. Biol., 20, 146–159 (2013), https://doi.org/10.1016/j.chembiol.2012.12.006.
D. Gu, H. Tang, J. Wu, et al., “Targeting Bruton tyrosine kinase using non-covalent inhibitors in B cell malignancies,” J. Hematol. Oncol., 14, 40 (2021), https://doi.org/10.1186/s13045-021-01049-7.
O. Crespo, S. C. Kang, R. Daneman, et al., “Tyrosine kinase inhibitors ameliorate autoimmune encephalomyelitis in a mouse model of multiple sclerosis,” J. Clin. Immunol., 31, 1010–1020 (2011), https://doi.org/10.1007/s10875-011-9579-6.
C. Menzfeld, M. John, D. van Rossum, et al., “Tyrphostin AG126 exerts neuroprotection in CNS inflammation by a dual mechanism: AG126 in autoimmunity and inflammation,” Glia, 63, 1083–1099 (2015), https://doi.org/10.1002/glia.22803.
S. Torke, R. Pretzsch, D. Häusler, et al., “Inhibition of Bruton’s tyrosine kinase interferes with pathogenic b-cell development in inflammatory CNS demyelinating disease,” Acta Neuropathol., 140, 535–548 (2020), https://doi.org/10.1007/s00401-020-02204-z.
S. Syed, N. Yonkers, C. LaGanke, et al., “Efficacy and safety of tolebrutinib in patients with highly active relapsing MS: Subgroup analysis of the phase 2b study,” Neurology, 96, S15, 2260 (2021).
A.García-Merino, “Bruton’s tyrosine kinase inhibitors: A new generation of promising agents for multiple sclerosis therapy,” Cells, 10, No. 10, 2560 (2021), https://doi.org/10.3390/cells10102560.
J. J. Crawford, A. R. Johnson, D. L. Misner, et al., “Discovery of GDC-0853: A potent, selective, and noncovalent Bruton’s tyrosine kinase inhibitor in early clinical development,” J. Med. Chem., 61, 2227–2245 (2018), https://doi.org/10.1021/acs.jmedchem.7b01712.
X. Montalban, D. L. Arnold, M. S. Weber, et al., “Placebo-controlled trial of an oral BTK inhibitor in multiple sclerosis,” N. Engl. J. Med., 380, 2406–2417 (2019), https://doi.org/10.1056/NEJMoa1901981.
S. Dhillon, “Orelabrutinib: First approval,” Drugs, 81, 503–507 (2021), https://doi.org/10.1007/s40265-021-01482-5.
L. J. Crofford, L. E. Nyhoff, J. H. Sheehan, et al., “The role of Bruton’s tyrosine kinase in autoimmunity and implications for therapy,” Expert Rev. Clin. Immunol., 12, 763–773 (2016), https://doi.org/10.1586/1744666X.2016.1152888.
A. Steinmaurer, I. Wimmer, T. Berger, et al., “Bruton’s tyrosine kinase inhibition in the treatment of preclinical models and multiple sclerosis,” Curr. Pharm. Des., 28, No. 6, 437–444 (2022), https://doi.org/10.2174/1381612827666210701152934.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Nevrologii i Psikhiatrii imeni S. S. Korsakova, Vol. 122, No. 7, Iss. 2, pp. 27–30, July, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boyko, A.N. Tyrosine Kinases: Targets for Epigenetic Influences and a New Direction in the Treatment of Multiple Sclerosis. Neurosci Behav Physi 53, 333–336 (2023). https://doi.org/10.1007/s11055-023-01430-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-023-01430-8