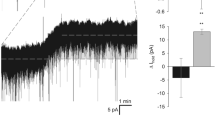
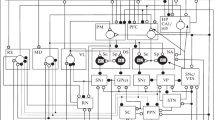
Recent studies have significantly expanded our understanding of the functions of GABAergic interneurons in cortical neural networks. Interneurons of specific classes are involved in generating interictal activity in the cortex not only in certain types of pathology, but also in conditions in which inhibition is mediated mainly via GABAB receptors. Interictal activity consists of high-amplitude spikes, where a short excitatory phase is followed by a long inhibitory phase occurring almost simultaneously in different parts of the cortex. Highamplitude spikes reflect the synchronous action of excitatory neurons in a local area, while synchronous activity in remote areas is determined by feedback between pyramidal cells and interneurons, when the activity of a large mass of neurons occurs simultaneously within a narrow time interval. Synchronization of interictal spikes involves Martinotti cells, as well as parvalbumin, neurogliaform, and vasoactive intestinal peptide-expressing interneurons, which, as experimental data show, also inhibit via GABAB receptors. Several mechanisms are now known which synchronize neuron activity in cortical neural networks: via electrical connections, volume conduction, and synaptic feedback – both between pyramidal neurons and interneurons and between interneurons. We propose that the mechanism of synchronization of interictal spikes in cortical neural networks operates as follows. This mechanism appears to operate in the same way both in local neural networks and over distances. When excitation occurs, it is followed by inhibition mediated by feedback; this limits the excitation period and thus creates a time window for integration, and this also occurs in neighboring cortical neural networks. At the initial stage, the amplitudes of interictal spikes are small and nonsimultaneous in different parts of the cortex. As time progresses, ever more pyramidal neurons become active during the time window, thus increasing the amplitude of the interictal spike, in turn increasing inhibition. Increased inhibition due to feedback ultimately begins to affect neighboring neural networks, with the result that interictal spikes appear almost simultaneously in different parts of the cortex. This produces a significant lengthening of postspike inhibition, as inhibition within a neural network is supplemented by inhibition from neighbors via inhibitory feedback.
Similar content being viewed by others
References
V. G. Marchenko and M. I. Zaichenko, “Dynamics of the spatial synchronization of epileptiform discharges in the rat neocortex,” Zh. Vyssh. Nerv. Deyat., 65, No. 1, 113 (2015).
V. G. Marchenko and K. A. Saltykov, “Synchronization mechanisms in local neural networks in the neocortex. Simulation and experimental studies,” Zh. Vyssh. Nerv. Deyat., 60, No. 1, 80 (2010).
V. G. Marchenko, M. P. Rysakova, and M. I. Zaichenko, “Distribution of internal electrical fields as a possible mechanism of synchronization of interictal spikes in the rat neocortex,” Zh. Vyssh. Nerv. Deyat., 68, No. 2, 250 (2018).
R. Aronoff, F. Matyas, C. Mateo, et al., “Long-range connectivity of mouse primary somatosensory barrel cortex,” Eur. J. Neurosci., 31, No. 12, 2221 (2010).
G. A. Ascoli, L. Alonso-Nanclares, S. A. Anderson, et al., “Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex,” Nat. Rev. Neurosci., 9, No. 7, 557 (2008).
M. Avoli, “Mechanisms of epileptiform synchronization in cortical neuronal networks,” Curr. Med. Chem., 21, No. 6, 653 (2014).
M. Avoli, M. de Curtis, V. Gnatkovsky, et al., “Specifi c imbalance of excitatory/inhibitory signaling establishes seizure onset pattern in temporal lobe epilepsy,” J. Neurophysiol., 115, No. 6, 3229–3237 (2016).
M. Barbarosie and M. Avoli, “CA3-driven hippocampal-entorhinal loop controls rather than sustains in vitro limbic seizures,” J. Neurosci., 17, No. 23, 9308–9314 (1997).
L. S. Benardo, “Separate activation of fast and slow inhibitory postsynaptic potentials in rat neocortex in vitro,” J. Physiol., 476, 203–215 (1994).
T. K. Berger, G. Silberberg, R. Perin, and H. Markram, “Brief bursts self-inhibit and correlate the pyramidal network,” PLoS Biol., 8, No. 9, e1000473 (2010).
H. Bink, M. Sedigh-Sarvestani, I. Fernandez-Lamo, et al., “Spatiotemporal evolution of focal epileptiform activity from surface and laminar field recordings in cat neocortex,” J. Neurophysiol., 119, No. 6, 2068–2081 (2018).
A. S. Bohannon and J. J. Hablitz, “Optogenetic dissection of roles of specific cortical interneuron subtypes in GABAergic network synchronization,” J. Physiol., 596, No. 5, 901–919 (2018).
S. A. Booker, D. Althof, and C. E. Degro, et al., “Differential surface density and modulatory effects of presynaptic GABAB receptors in hippocampal cholecystokinin and parvalbumin basket cells,” Brain Struct. Funct., 222, No. 8, 3677–3690 (2017).
S. A. Booker, D. Loreth, A. L. Gee, et al., “Postsynaptic GABABRs inhibit L-type calcium channels and abolish long-term potentiation in hippocampal somatostatin interneurons,” Cell Rep., 22, No. 1, 36–43 (2018).
A. Caputi, S. Melzer, M. Michael, and H. Monyer, “The long and short of GABAergic neurons,” Curr. Opin. Neurobiol., 23, No. 2, 179–186 (2013).
L. Chauvière, T. Doublet, A. Ghestem, et al., “Changes in interictal spike features precede the onset of temporal lobe epilepsy,” Ann. Neurol., 71, No. 6, 805–814 (2012).
R. Chittajallu, K. A. Pelkey, and C. J. McBain, “Neurogliaform cells dynamically regulate somatosensory integration via synapse-specific modulation,” Nat. Neurosci., 16, No. 1, 13–15 (2013).
D. Contreras, I. Timofeev, and M. Steriade, “Mechanisms of long-lasting hyperpolarizations underlying slow sleep oscillations in cat corticothalamic networks,” J. Physiol., 494, No. 1, 251–264 (1996).
M. T. Craig, E. W. Mayne, B. Bettler, et al., “Distinct roles of GABAB1a- and GABAB1b-containing GABAB receptors in spontaneous and evoked termination of persistent cortical activity,” J. Physiol., 591, No. 4, 835–843 (2013).
M. T. Craig and C. J. McBain, “The emerging role of GABAB receptors as regulators of network dynamics: fast actions from a ‘slow’ receptor,” Curr. Opin. Neurobiol., 26, 15–21 (2014).
M. T. Craig and C. J. McBain, “Navigating the circuitry of the brain’s GPS system: Future challenges for neurophysiologists,” Hippocampus, 25, No. 6, 736–743 (2015), https://doi.org/10.1002/hipo.22456.
M. D’Antuono, J. Louvel, R. Köhling, et al., “GABAA receptor-dependent synchronization leads to ictogenesis in the human dysplastic cortex,” Brain, 127, No. 7, 1626–1640 (2004).
M. R. Deans, J. R. Gibson, C. Sellitto, et al., “Synchronous activity of inhibitory networks in neocortex requires electrical synapses containing connexin36,” Neuron, 31, No. 3, 477–485 (2001).
M. de Curtis and G. Avanzini, “Interictal spikes in focal epileptogenesis,” Prog. Neurobiol., 63, No. 5, 541–567 (2001).
M. de Curtis and M. Avoli, “GABAergic networks jump-start focal seizures,” Epilepsia, 57, No. 5, 679–687 (2016).
M. de Curtis, C. Radici, and M. Forti, “Cellular mechanisms underlying spontaneous interictal spikes in an acute model of focal cortical epileptogenesis,” Neuroscience, 88, No. 1, 107–117 (1999).
J. DeFelipe, P. L. López-Cruz, R. Benavides-Piccione, et al., “New insights into the classifi cation and nomenclature of cortical GABAergic interneurons,” Nat. Rev. Neurosci., 14, No. 3, 202–216 (2013).
T. Dorn and O. W. Witte, “Refractory periods following interictal spikes in acute experimentally induced epileptic foci,” Electroencephalogr. Clin. Neurophysiol., 94, No. 1, 80–85 (1995).
E. E. Fanselow and B. W. Connors, “The roles of somatostatin-expressing (GIN) and fast-spiking inhibitory interneurons in UPDOWN states of mouse neocortex,” J. Neurophysiol., 104, No. 2, 596–606 (2010).
E. E. Fanselow, K. A. Richardson, and B. W. Connors, “Selective, state-dependent activation of somatostatin-expressing inhibitory interneurons in mouse neocortex,” J. Neurophysiol., 100, No. 5, 2640–2652 (2008).
D. Feldmeyer, M. Brecht, F. Helmchen, et al., “Barrel cortex function,” Prog. Neurobiol., 103, 3–27 (2013).
D. Feldmeyer, G. Qi, V. Emmenegger, and J. F. Staiger, “Inhibitory interneurons and their circuit motifs in the many layers of the barrel cortex,” Neuroscience, 368, 132–151 (2018).
E. Fino and R. Yuste, “Dense inhibitory connectivity in neocortex,” Neuron, 69, No. 6, 1188–1203 (2011).
C. M. Funk, K. Peelman, M. Bellesi, et al., “Role of somatostatin-positive cortical interneurons in the generation of sleep slow waves,” J. Neurosci., 37, No. 38, 9132–9148 (2017).
F. Fröhlich and D. McCormick, “Endogenous electric fields may guide neocortical network activity,” Neuron, 67, No. 1, 129–143 (2010).
M. Galarreta and S. Hestrin, “Spike transmission and synchrony detection in networks of GABAergic interneurons,” Science, 292, No. 5525, 2295–2299 (2001).
L. J. Gentet, M. Avermann, F. Matyas, et al., “Membrane potential dynamics of GABAergic Neurons in the barrel cortex of behaving mice,” Neuron, 65, No. 3, 422–435 (2010).
L. J. Gentet, Y. Kremer, H. Taniguchi, et al., “Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex,” Nat. Neurosci., 15, No. 4, 607–612 (2012).
L. B. Gerrard, M. L. S. Tantirigama, and J. M. Bekkers, “Pre- and postsynaptic activation of gabab receptors modulates principal cell excitation in the piriform cortex,” Front. Cell. Neurosci, 12, 28 (2018).
J. R. Gibson, M. Beierlein, and B. W. Connors, “Functional properties of electrical synapses between inhibitory interneurons of neocortical layer 4,” J. Neurophysiol., 93, No. 1, 467–480 (2005).
J. H. Goldberg, C. O. Lacefield, and R. Yuste, “Global dendritic calcium spikes in mouse layer 5 low threshold spiking interneurones: implications for control of pyramidal cell bursting,” J. Physiol., 558 (Pt 2), 465–478 (2004).
Y. Gonchar, L. Pang, B. Malitschek, et al., “Subcellular localization of GABA(B) receptor subunits in rat visual cortex,” J. Comp. Neurol., 431, No. 2, 182–197 (2001).
C. M. Gray and W. Singer, “Stimulus-specifi c neuronal oscillations in orientation columns of cat visual cortex,” Proc. Natl. Acad. Sci. USA, 86, No. 5, 1698–1702 (1989).
M. M. Hilscher, R. N. Leão, S. J. Edwards, et al., “Chrna2-Martinotti cells synchronize layer 5 type A pyramidal cells via rebound excitation,” PLoS Biol., 15, No. 2, e2001392 (2017).
H. Hu and A. Agmon, “Properties of precise fi ring synchrony between synaptically coupled cortical interneurons depend on their mode of coupling,” J. Neurophysiol., 114, No. 1, 624–637 (2015).
J. S. Isaacson and M. Scanziani, “How inhibition shapes cortical activity,” Neuron, 72, No. 2, 231–243 (2011), https://doi.org/10.1016/j.neuron.2011.09.027.
X. Jiang, G. Wang, and J. Zhu, “The organization of two new cortical interneuronal circuits,” Nat. Neurosci., 16, No. 2, 210–218 (2013).
X. Jiang, S. Shen, C. R. Cadwell, et al., “Principles of connectivity among morphologically defined cell types in adult neocortex,” Science, 350, No. 6264, aac9462 (2015).
C. Kapfer, L. L. Glickfeld, B. V. Atallah, and M. Scanziani, “Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex,” Nat. Neurosci., 10, 743–753 (2007).
A. Karagiannis, T. Gallopin, C. Dávid, et al., “Classification of NPY-expressing neocortical interneurons,” J. Neurosci., 29, No. 11, 3642–3659 (2009).
M. M. Karnani, J. Jackson, I. Ayzenshtat, et al., “Opening holes in the blanket of inhibition: localized lateral disinhibition by VIP interneurons,” J. Neurosci., 36, 3471–3480 (2016).
M. M. Karnani, J. Jackson, I. Ayzenshtat, et al., “Cooperative subnetworks of molecularly similar interneurons in mouse neocortex,” Neuron, 90, No. 1, 86–100 (2016).
F. Karube, Y. Kubota, and Y. Kawaguchi, “Axon branching and synaptic bouton phenotypes in GABAergic nonpyramidal cell subtypes,” J. Neurosci., 24, No. 12, 2853–2865 (2004).
Y. Kawaguchi, F. Karube, and Y. Kubota, “Dendritic branch typing and spine expression patterns in cortical nonpyramidal cells,” Cereb. Cortex, 16, 696–711 (2006), https://doi.org/10.1093/cercor/bhj015.
Y. Kawaguchi and Y. Kubota, “Physiological and morphological identification of somatostatin- or vasoactive intestinal polypeptide- containing cells among GABAergic cell subtypes in rat frontal cortex,” J. Neurosci., 16, No. 8, 2701–2715 (1996).
Y. Kawaguchi and Y. Kubota, “GABAergic cell subtypes and their synaptic connections in rat frontal cortex,” Cereb. Cortex, 7, No. 6, 476–486 (1997).
Y. Kawaguchi and T. Shindou, “Noradrenergic excitation and inhibition of gabaergic cell types in rat frontal cortex,” J. Neurosci., 18, No. 17, 6963–6976 (1998).
C. Koelbl, M. Helmstaedter, J. Lübke, and D. Feldmeyer, “A barrel-related interneuron in layer 4 of rat somatosensory cortex with a high intrabarrel connectivity,” Cereb. Cortex, 25, 713–725 (2015), https://doi.org/10.1093/cercor/bht263.
R. Köhling, M. D’Antuono, R. Benini, et al., “Hypersynchronous ictal onset in the perirhinal cortex results from dynamic weakening in inhibition,” Neurobiol. Dis., 87, 1–10 (2016).
M. M. Kohl and O. Paulsen, “The roles of GABAB receptors in cortical network activity,” Adv. Pharmacol., 58, 205–229 (2010).
A. M. Large, N. W. Vogler, S. Mielo, and A. M. Oswald, “Inhibition by somatostatin interneurons in olfactory cortex,” Front. Neural Circuits, 10, 62 (2016).
S. H. Lee, J. Hjerling-Leffler, E. Zagha, et al., “The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors,” J. Neurosci., 30, No. 50, 16796–16808 (2010).
S. Lee, I. Kruglikov, Z. J. Huang, et al., “A disinhibitory circuit mediates motor integration in the somatosensory cortex,” Nat. Neurosci., 16, No. 11, 1662–1670 (2013).
A. J. Lee, G. Wang, X. Jiang, et al., “Canonical organization of layer 1 neuron-led cortical inhibitory and disinhibitory interneuronal circuits,” Cereb. Cortex, 25, No. 8, 2114–2126 (2015).
J.-F. Léger, E. A. Stern, A. Aertsen, and D. Heck, “Synaptic integration in rat frontal cortex shaped by network activity,” J. Neurophysiol., 93, 281–293 (2005).
L. Librizzi and M. de Curtis, “Epileptiform ictal discharges are prevented by periodic interictal spiking in the olfactory cortex,” Ann. Neurol., 53, No. 3, 382–389 (2003).
D. H. Lim, M. H. Mohajerani, J. Ledue, et al., “In vivo large-scale cortical mapping using channelrhodopsin-2 stimulation in transgenic mice reveals asymmetric and reciprocal relationships between cortical areas,” Front. Neural Circuits, 6, 11 (2012).
K. P. Lillis, M. A. Kramer, J. Mertz, et al., “Pyramidal cells accumulate chloride at seizure onset,” Neurobiol. Dis., 47, No. 3, 358–366 (2012).
L. Liu, W. Ito, and A. Morozov, “GABAb receptor mediates opposing adaptations of GABA release from two types of prefrontal interneurons after observational fear,” Neuropsychopharmacology, 42, No. 6, 1272–1283 (2017).
Y. Ma, H. Hu, A. S. Berrebi, et al., “Distinct subtypes of somatostatin- containing neocortical interneurons revealed in transgenic mice,” J. Neurosci., 26, No. 19, 5069–5082 (2006).
J. G. Mancilla, T. J. Lewis, D. J. Pinto, et al., “Synchronization of electrically coupled pairs of inhibitory interneurons in neocortex,” J. Neurosci., 27, 2058–2073 (2007).
E. O. Mann, C. A. Radcliffe, and O. Paulsen, “Hippocampal gamma- frequency oscillations: from interneurones to pyramidal cells, and back,” J. Physiol., 562, Pt. 1, 55–63 (2005).
T. Mao, D. Kusefoglu, B. M. Hooks, et al., “Long-range neuronal circuits underlying the interaction between sensory and motor cortex,” Neuron, 72, No. 1, 111–123 (2011).
F. Matyas, V. Sreenivasan, F. Marbach, et al., “Motor control by sensory cortex,” Science, 330, 1240 (2010).
A. J. McDonald, F. Mascagni, and J. F. Muller, “Immunocytochemical localization of GABABR1 receptor subunits in the basolateral amygdala,” Brain Res., 1018, 147–158 (2004), https://doi.org/10.1016/j.brainres.2004.
S. Melzer, M. Michael, A. Caputi, et al., “Long-range-projecting GABAergic neurons modulate inhibition in hippocampus and entorhinal cortex,” Science, 335, 1506–1510 (2012).
M. Morishima, K. Kobayashi, S. Kato, et al., “Segregated excitatory- inhibitory recurrent subnetworks in layer 5 of the rat frontal cortex,” Cereb. Cortex, 27, No. 12, 5846–5857 (2017).
S. F. Muldoon, V. Villette, T. F. Tressard, et al., “GABAergic inhibition shapes interictal dynamics in awake epileptic mice,” Brain, 138, Pt. 10, 2875–2890 (2015).
M. Murayama, E. Pérez-Garci, T. Nevian, et al., “Dendritic encoding of sensory stimuli controlled by deep cortical interneurons,” Nature, 457, No. 7233, 1137–1141 (2009).
A. Naka and H. Adesnik, “inhibitory circuits in cortical layer 5,” Front. Neural Circuits, 10, 35 (2016).
G. T. Neske, S. L. Patrick, and B. W. Connors, “Contributions of diverse excitatory and inhibitory neurons to recurrent network activity in cerebral cortex,” J. Neurosci., 35, No. 3, 1089–1105 (2015).
R. A. Nicoll, R. C. Malenka, and J. A. Kauer, “Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system,” Physiol. Rev., 70, No. 2, 513–565 (1990).
M. J. Nigro, Y. Hashikawa-Yamasaki, and B. Rudy, “Diversity and connectivity of layer 5 somatostatin-expressing interneurons in the mouse barrel cortex,” J. Neurosci., 38, No. 7, 1622–1633 (2018).
S. Oláh, M. Füle, G. Komlósi, et al., “Regulation of cortical microcircuits by unitary GABAergic volume transmission,” Nature, 461, No. 7268, 1278–1281 (2009).
S. Oláh, G. Komlósi, J. Szabadics, et al., “Output of neurogliaform cells to various neuron types in the human and rat cerebral cortex,” Front. Neural Circuits, 1, 4 (2007).
T. Otsuka and Y. Kawaguchi, “Cortical inhibitory cell types differentially form intralaminar and interlaminar subnetworks with excitatory neurons,” J. Neurosci., 29, No. 34, 10533–10540 (2009).
L. Palmer, M. Murayama, and M. Larkum, “Inhibitory regulation of dendritic activity in vivo,” Front. Neural Circuits, 6, 26 (2012).
L. M. Palmer, J. M. Schulz, S. C. Murphy, et al., “The cellular basis of GABA(B)-mediated interhemispheric inhibition,” Science, 335, No. 6071, 989–993 (2012).
G. Panuccio, G. Curia, A. Colosimo, et al., “Epileptiform synchronization in the cingulate cortex,” Epilepsia, 50, No. 3, 521–536 (2009).
C. C. Petersen and S. Crochet, “Synaptic computation and sensory processing in neocortical layer 2/3,” Neuron, 78, No. 1, 28–48 (2013).
C. K. Pfeffer, M. Xue, M. He, et al., “Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons,” Nat. Neurosci., 16, No. 8, 1068–1076 (2013).
C. J. Price, B. Cauli, E. R. Kovacs, et al., “Neurogliaform neurons form a novel inhibitory network in the hippocampal CA1 area,” J. Neurosci., 25, No. 29, 6775–6786 (2005).
C. J. Price, R. Scott, D. A. Rusakov, and M. Capogna, “GABA(B) receptor modulation of feedforward inhibition through hippocampal neurogliaform cells,” J. Neurosci., 28, No. 27, 6974–6982 (2008).
D. A. Prince, “Inhibition in ‘epileptic’ neurons,” Exp. Neurol., 21, No. 3, 307–321 (1968).
A. Pronneke, B. Scheuer, R. J. Wagener, et al., “Characterizing VIP Neurons in the barrel cortex of VIPcre/tdTomato mice reveals layer- specific differences,” Cereb. Cortex, 25, No. 12, 4854–4868 (2015).
M. Rocco-Donovan, R. L. Ramos, S. Giraldo, and J. C. Brumberg, “Characteristics of synaptic connections between rodent primary somatosensory and motor cortices,” Somatosens. Mot. Res., 28, No. 3–4, 63–72 (2011).
A. K. Roopun, J. D. Simonotto, M. L. Pierce, et al., “A nonsynaptic mechanism underlying interictal discharges in human epileptic neocortex,” Proc. Natl. Acad. Sci. USA, 107, No. 1, 338–343 (2010).
B. Rudy, G. Fishell, S. Lee, and J. Hjerling-Leffler, “Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons,” Dev. Neurobiol., 71, No. 1, 45–61 (2011).
H. R. Sabolek, W. B. Swiercz, Lillis K et al.,”A candidate mechanism underlying the variance of interictal spike propagation,” J. Neurosci., 32, No. 9, 3009–3021 (2012).
R. Saffari, Z. Teng, M. Zhang, et al., “NPY+-, but not PV+-GABAergic neurons mediated long-range inhibition from infra- to prelimbic cortex,” Transl. Psychiatry, 6, e736 (2016).
D. B. Salkoff, E. Zagha, Ö. Yüzgeç, and D. A. McCormick, “Synaptic mechanisms of tight spike synchrony at gamma frequency in cerebral cortex,” J. Neurosci., 35, No. 28, 10236–10251 (2015).
C. A. Schevon, S. K. Ng, J. Cappell, et al., “Microphysiology of epileptiform activity in human neocortex,” J. Clin. Neurophysiol., 25, No. 6, 321–330 (2008).
I. Scheyltjens and L. Arckens, “The current status of somatostatin- interneurons in inhibitory control of brain function and plasticity,” Neural Plast., 2016, 8723623 (2016).
G. Silberberg and H. Markram, “Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells,” Neuron, 53, 735–746 (2007).
A. Simon, S. Oláh, G. Molnár, et al., “Gap-junctional coupling between neurogliaform cells and various interneuron types in the neocortex,” J. Neurosci., 25, No. 27, 6278–6285 (2005).
M. Steriade, A. Nuñez, and F. Amzica, “A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components,” J. Neurosci., 13, No. 8, 3252–3265 (1993).
G. Tamás, A. Lorincz, A. Simon, and J. Szabadics, “Identifi ed sources and targets of slow inhibition in the neocortex,” Science, 299, No. 5614, 1902–1905 (2003).
I. Timofeev, F. Grenier, and M. Steriade, “The role of chloride-dependent inhibition and the activity of fast-spiking neurons during cortical spike-wave electrographic seizures,” Neuroscience, 114, No. 4, 1115–1132 (2002).
R. Tremblay, S. Lee, and B. Rudy, “GABAergic Interneurons in the neocortex: from cellular properties to circuits,” Neuron, 91, No. 2, 260–292 (2016).
A. J. Trevelyan, D. Sussillo, B. O. Watson, and R. Yuste, “Modular propagation of epileptiform activity: evidence for an inhibitory veto in neocortex,” J. Neurosci., 26, No. 48, 12447–1255 (2006).
A. Trevelyan, D. Sussillo, and R. Yuste, “Feedforward inhibition contributes to the control of epileptiform propagation speed,” J. Neurosci., 27, No. 13, 3383–3387 (2007).
J. Urban-Ciecko and A. L. Barth, “Somatostatin-expressing neurons in cortical networks,” Nat. Rev. Neurosci., 17, No. 7, 401–409 (2016).
J. Urban-Ciecko, E. E. Fanselow, and A. L. Barth, “Neocortical somatostatin neurons reversibly silence excitatory transmission via GABAb receptors,” Curr. Biol., 25, No. 6, 722–731 (2015).
M. P. Vanni and T. H. Murphy, “Mesoscale transcranial spontaneous activity mapping in GCaMP3 transgenic mice reveals extensive reciprocal connections between areas of somatomotor cortex,” J. Neurosci., 34, No. 48, 15931–15946 (2014).
E. D. Vickers, C. Clark, D. Osypenko, et al., “Parvalbumininterneuron output synapses show spike-timing-dependent plasticity that contributes to auditory map remodeling,” Neuron, 99, No. 4, 720–735.e6 (2018).
J. Veit, R. Hakim, M. P. Jadi, et al., “Cortical gamma band synchronization through somatostatin interneurons,” Nat. Neurosci., 20, No. 7, 951–959 (2017).
L. Wang and A. Maffei, “Inhibitory plasticity dictates the sign of plasticity at excitatory synapses,” J. Neurosci., 34, No. 4, 1083–1093 (2014).
Y. Wang, F. B. Neubauer, H. R. Lüscher, and K. Thurley, “GABAB receptor-dependent modulation of network activity in the rat prefrontal cortex in vitro,” Eur. J. Neurosci., 31, No. 9, 1582–1594 (2010).
Y. Wang, M. Toledo-Rodriguez, A. Gupta, et al., “Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat,” J. Physiol., 561, No. 1, 65–90 (2004).
M. Wehr and A. M. Zador, “Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex,” Nature, 426, 442–446 (2003).
O. W. Witte, “Physiological basis of pathophysiological brain rhythms,” Acta Neurobiol. Exp. (Wars.), 60, No. 2, 289–297 (2000).
L. Yekhlef, G. L. Breschi, L. Lagostena, et al., “Selective activation of parvalbumin- or somatostatin-expressing interneurons triggers epileptic seizurelike activity in mouse medial entorhinal cortex,” J. Neurophysiol., 113, No. 5, 1616–1630 (2015).
I. Yavorska and M. Wehr, “Somatostatin-expressing inhibitory interneurons in cortical circuits,” Front. Neural Circuits, 10, 76 (2016).
J. Zhu, M. Jiang, M. Yang, et al., “Membrane potential-dependent modulation of recurrent inhibition in rat neocortex,” PLoS Biol., 9, No. 3, e1001032 (2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Uspekhi Fiziologicheskikh Nauk, Vol. 53, No. 1, pp. 52–75, January–March, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marchenko, V.G., Zaichenko, M.I. GABAB Inhibition through Feedback Is Involved in the Synchronization of Interictal Spikes in the Cortex. Neurosci Behav Physi 52, 1506–1523 (2022). https://doi.org/10.1007/s11055-023-01381-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-023-01381-0