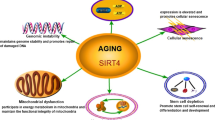
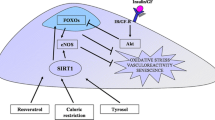
The sirtuin family of proteins (SIRT proteins) are involved in DNA repair, chromatin remodeling, epigenetic regulation of the expression of metabolism genes, the antioxidant system, apoptosis, immuno- and neurogenesis, etc. The aim of this review is to analyze the geroprotective properties of sirtuins in normal and age-associated pathologies. SIRT1, 2, 3, 4, and 6 contribute to increases in life expectancy. SIRT1, 2, 6, and 7 slow cellular aging and maintain the stem cell pool. Sirtuins are potential targets for the treatment of neurodegenerative, oncological, and cardiovascular diseases, metabolic syndrome, and diabetes mellitus. All these diseases are in most cases characteristic of elderly and old people, so the geroprotective effects of sirtuins, realized at the molecular and cellular levels, may play an important role in the treatment of these conditions.
Similar content being viewed by others
References
A. E. Pukhalskaia, A. S. Diatlova, N. S. Linkova, et al., “Sirtuins as possible predictors of ageing and the development of Alzheimer’s disease: verifi cation in the hippocampus and saliva,” Bull. Exp. Biol. Med., 169, No. 6, 769–772 (2020).
A. E. Pukhalskaia, A. S. Diatlova, N. S. Linkova, and I. M. Kvetnoy, “Sirtuins: role and regulation of oxidative stress and the pathogenesis of neurodegenerative diseases,” Usp. Fiziol. Nauk., 52, No. 1, 90–104 (2021).
B. H. Ahn, H. S. Kim, S. Song, et al., “A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis,” Proc. Natl. Acad. Sci. USA, 105, No. 38, 14447–14452 (2008), https://doi.org/10.1073/pnas.0803790105.
M. Almeida and R. M. Porter, “Sirtuins and FoxOs in osteoporosis and osteoarthritis,” Bone, 121, 284–292 (2019), https://doi.org/10.1016/j.bone.2019.01.018.
T. Anwar, S. Khosla, and G. Ramakrishna, “Increased expression of SIRT2 is a novel marker of cellular senescence and is dependent on wild type p53 status,” Cell Cycle, 15, No. 14, 1883–1897 (2016), https://doi.org/10.1080/15384101.2016.1189041.
H. Bai, S. Post, P. Kang, and M. Tatar, “Drosophila longevity assurance conferred by reduced insulin receptor substrate chico partially requires d4eBP,” PLoS One, 10, e0134415 (2015), https://doi.org/10.1371/journal.pone.0134415.
A. Benigni, P. Cassis, S. Conti, et al., “Sirt3 deficiency shortens life span and impairs cardiac mitochondrial function rescued by Opa1 gene transfer,” Antioxid. Redox. Signal., 31, No. 17, 1255–1271 (2019), https://doi.org/10.1089/ars.2018.7703.
N. Braidy, G. J. Guillemin, H. Mansour, et al., “Age related changes in NAD+ metabolism oxidative stress and SIRT1 activity in Wistar rats,” PLoS One, 6, No. 4: e19194 (2011), https://doi.org/10.1371/journal.pone.0019194.
N. Braidy, A. Poljak, R. Grant, et al., “Differential expression of sirtuins in the aging rat brain,” Front. Cell. Neurosci., 9, 167 (2015), https://doi.org/10.3389/fncel.2015.00167.
A. Brunet, L. B. Sweeney, J. F. Sturgill, et al., “Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase,” Science, 303, No. 5666), 2011–2015 (2004), https://doi.org/10.1126/science.1094637.
K. Burkewitz, Y. E. Zhang, and W. B. Mair, “AMPK at the nexus of energetics and aging,” Cell Metab., 20, 10–25 (2014), https://doi.org/10.1016/j.cmet.2014.03.002.
C. Burnett, S. Valentini, F. Cabreiro, et al., “Absence of effects of Sir2 overexpression on lifespan in, “elegans and Drosophila,” Nature, 477, 482–485 (2011), https://doi.org/10.1038/nature10296.
A. Cardinale, M. C. de Stefano, C. Mollinari, et al., “Biochemical characterization of sirtuin 6 in the brain and its involvement in oxidative stress response,” Neurochem. Res., 40, No. 1, 59–69 (2015), https://doi.org/10.1007/s11064-014-1465-1.
C. Chen, M. Zhou, Y. Ge, and X. Wang, “SIRT1 and aging related signaling pathways,” Mech. Aging Dev., 187, 111215 (2020), https://doi.org/10.1016/j.mad.2020.111215.
S. H. Cho, J. A. Chen, F. Sayed, et al., “SIRT1 deficiency in microglia contributes to cognitive decline in aging and neurodegeneration via epigenetic regulation of IL-1 beta,” J. Neurosci., 35, 807–818 (2015), https://doi.org/10.1523/JNEUROSCI.2939-14.2015.
N. D’Onofrio, M. Vitiello, R. Casale, et al., “Sirtuins in vascular diseases: emerging roles and therapeutic potential,” Biochim. Biophys. Acta, 1852, No. 7, 1311–1322 (2015), https://doi.org/10.1016/j.bbadis.2015.03.001.
W. Dang, “The controversial world of sirtuins,” Drug Discov. Today Technol., 12, e9–e17 (2014), https://doi.org/10.1016/j.ddtec.2012.08.003.
M. A. Deprez, E. Eskes, J. Winderickx, and T. Wilms, “The TORC1-Sch9 pathway as a crucial mediator of chronological lifespan in the yeast Saccharomyces cerevisiae,” FEMS Yeast Res., 18, 18 (2018), https://doi.org/10.1093/femsyr/foy048.
A. J. Donato, K. A. Magerko, B. R. Lawson, et al., “SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans,” J. Physiol., 589, 4545–4554 (2011), https://doi.org/10.1113/jphysiol.2011.211219.
J. Du, Y. Zhou, X. Su, et al., “Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase,” Science, 334, 806–809 (2011), https://doi.org/10.1126/science.1207861.
J. R. Edwards, D. S. Perrien, N. Fleming, et al., “Silent information regulator (Sir)T1 inhibits NF-κB signaling to maintain normal skeletal remodeling,” J. Bone Miner. Res, 28, No. 4, 960–969 (2013), https://doi.org/10.1002/jbmr.1824.
E. F. Fang, S. Lautrup, Y. J. Hou, et al., “NAD+ in aging: molecular mechanisms and translational implications,” Trends Mol. Med., 23, 899–916 (2017), https://doi.org/10.1016/j.molmed.2017.08.001.
L. W. Finley, A. Carracedo, J. Lee, et al., “SIRT3 opposes reprogramming of Cancer Cell metabolism through HIF1α destabilization,” Cancer Cell, 19, No. 3, 416–428 (2011), https://doi.org/10.1016/j.ccr.2011.02.014.
R. A. Frye, “Phylogenetic classifi cation of prokaryotic and eukaryotic Sir2-like proteins,” Biochem. Biophys. Res. Commun, 273, No. 2, 793–798 (2000), https://doi.org/10.1006/bbrc.2000.3000.
A. García-Aguilar, C. Guillén, M. Nellist, et al., “TSC2 N-terminal lysine acetylation status affects to its stability modulating mTORC1 signaling and autophagy,” Biochim. Biophys. Acta, 1863, No. 11, 2658–2667 (2016), https://doi.org/10.1016/j.bbamcr.2016.08.006.
H. S. Ghosh, M. McBurney, and P. D. Robbins, “SIRT1 negatively regulates the mammalian target of rapamycin,” PLoS One, 5, No. 2, e9199 (2010), https://doi.org/10.1371/journal.pone.0009199.
J. Guo, M. Shao, F. Lu, et al., “Role of SIRT1 plays in nucleus pulposus cells and intervertebral disc degeneration,” Spine (Phila Pa 1976), 49, No. 13, E757–E766 (2017), https://doi.org/10.1097/BRS.0000000000001954.
M. C. Haigis and L. P. Guarente, “Mammalian sirtuins-emerging roles in physiology, aging, and calorie restriction,” Genes Dev., 20, 2913–2921 (2006), https://doi.org/10.1101/gad.1467506.
M. C. Haigis, R. Mostoslavsky, K. M. Haigis, et al., “SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta Cells,” Cell, 126, 941–954 (2006), https://doi.org/10.1016/j.cell.2006.06.057.
P. Han, Z. Tang, J. Yin, et al., “Pituitary adenylate cyclase-activating polypeptide protects against β-amyloid toxicity,” Neurobiol. Aging, 35, No. 9, 2064–2071 (2014), https://doi.org/10.1016/j.neurobiolaging.2014.03.022.
M. D. Hirschey, T. Shimazu, E. “Jing, et al., “SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome,” Mol. Cell., 44, No. 2, 177–190 (2011), https://doi.org/10.1016/j.molcel.2011.07.019.
M. D. Hirschey, T. Shimazu, E. Goetzman, et al., “SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation,” Nature, 464, No. 7285), 121–125 (2010), https://doi.org/10.1038/nature08778.
Y. S. Hori, A. Kuno, R. Hosoda, and Y. Horio, “Regulation of FOXOs and p53 by SIRT1 modulators under oxidative stress,” PLoS One, 8, No. 9, e73875 (2013), https://doi.org/10.1371/journal.pone.0073875.
R. H. Houtkooper, E. Pirinen, and J. Auwerx, “Sirtuins as regulators of metabolism and healthspan,” Nat. Rev. Mol. Cell. Biol., 13, 225–238 (2012), https://doi.org/10.1038/nrm3293.
M. E. Hubbi, H. Hu, Kshitiz et al, “Sirtuin-7 inhibits the activity of hypoxia-inducible factors,” J. Biol. Chem., 288, No. 29, 20768–20775 (2013), https://doi.org/10.1074/jbc.M113.476903.
S. M. Jeon, “Regulation and function of AMPK in physiology and diseases,” Exp. Mol. Med., 48, e245 (2016), https://doi.org/10.1038/emm.2016.81.
E. S. Jung, H. Choi, and H. Song, et al., “p53-dependent SIRT6 expression protects Aβ42-induced DNA damage,” Sci. Rep., 6, 25628 (2016), https://doi.org/10.1038/srep25628.
M. Kaeberlein, M. McVey, and L. Guarente, “The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms,” Genes Dev., 13, No. 19, 2570–2580 (1999), https://doi.org/10.1101/gad.13.19.2570.
S. Kaluski, M. Portillo, and A. Besnard, et al., “Neuroprotective Functions for the Histone Deacetylase SIRT6,” Cell Rep., 18, No. 13, 3052–3062 (2017), https://doi.org/10.1016/j.celrep.2017.03.008.
Y. Kanfi, S. Naiman, and G. Amir, et al., “The sirtuin SIRT6 regulates lifespan in male mice,” Nature, 483, No. 7388, 218–221 (2012), https://doi.org/10.1038/nature10815.
C. L. Kao, L. K. Chen, Y. L. Chang, et al., “Resveratrol protects human endothelium from H2O2- induced oxidative stress and senescence via SIRT1 activation,” J. Atheroscler. Thromb, 17, 970–979 (2010), https://doi.org/10.5551/jat.4333.
A. A. Kendrick, M. Choudhury, S. M. Rahman, et al., “Fatty liver is associated with reduced SIRT3 activity and mitochondrial protein hyperacetylation,” Biochem. J., 433, No. 3, 505–514 (2011), https://doi.org/10.1042/BJ20100791.
B. K. Kennedy, M. Gotta, D. A. Sinclair, et al., “Redistribution of silencing proteins from telomeres to the nucleolus is associated with extension of life span in, “cerevisiae,” Cell, 89, No. 3, 381–391 (1997), https://doi.org/10.1016/s0092-8674(00)80219-6.
M. Kim, J. S. Lee, J. E. Oh, et al., “SIRT3 Overexpression Attenuates Palmitate-Induced Pancreatic β-Cell Dysfunction,” PLoS One, 10, No. 4, e0124744 (2015), https://doi.org/10.1371/journal.pone.0124744.
S. Kiran, T. Anwar, M. Kiran, and G. Ramakrishna, “Sirtuin 7 in cell proliferation, stress and disease: Rise of the Seventh Sirtuin!,” Cell. Signal., 27, No. 3, 673–682 (2015), https://doi.org/10.1016/j.cellsig.2014.11.026.
M. Kitada, Y. Ogura, and D. Koya, “The protective role of SIRT1 in vascular tissue: its relationship to vascular aging and atherosclerosis,” Aging (Albany NY), 8, No. 10, 2290–2307 (2016), 10.18632/aging.101068.
M. Kitada, Y. Ogura, I. Monno, and D. Koya, “Sirtuins and type 2 diabetes: role in inflammation, oxidative stress, and mitochondrial function,” Front. Endocrinol. (Lausanne), 10, 187 (2019), https://doi.org/10.3389/fendo.2019.00187.
M. A. Klein and J. M. Denu, “Biological and catalytic functions of sirtuin 6 as targets for small-molecule modulators,” J. Biol. Chem., 295, No. 32, 11021–11041 (2020), https://doi.org/10.1074/jbc.REV120.011438.
S. Kugel and R. Mostoslavsky, “Chromatin and beyond: the multitasking roles for SIRT6,” Trends Biochem. Sci., 39, No. 2, 72–81 (2014), https://doi.org/10.1016/j.tibs.2013.12.002.
S. Kugel, C. Sebastián, J. Fitamant, et al., “SIRT6 suppresses pancreatic cancer through control of Lin28b,” Cell, 165, No. 6, 1401–1415 (2016), https://doi.org/10.1016/j.cell.2016.04.033.
A. Lang, S. Grether-Beck, M. Singh, et al., “MicroRNA-15b regulates mitochondrial ROS production and the senescence-associated secretory phenotype through sirtuin 4/SIRT4,” Aging (Albany NY), 8, No. 3, 484–505 (2016), 10.18632/aging.100905.
I. R. Lanza, D. K. Short, and K. R. Short, et al., “Endurance exercise as a countermeasure for aging,” Diabetes, 57, No. 11, 2933–2942 (2008), https://doi.org/10.2337/db08-0349.
F. Li and L. Liu, “SIRT5 defi ciency enhances susceptibility to kainate-induced seizures and exacerbates hippocampal neurodegeneration not through mitochondrial antioxidant enzyme SOD2,” Front. Cell. Neurosci, 10, 171 (2016), https://doi.org/10.3389/fncel.2016.00171.
C. J. Lim, Y. M. Lee, S. G. Kang, et al., “Aquatide activation of SIRT1 reduces cellular senescence through a SIRT1-FOXO1-autophagy axis,” Biomol. Ther. (Seoul), 25, No. 5, 511–518 (2017), https://doi.org/10.4062/biomolther.2017.119.
C. H. Lin, J. Chen, B. Ziman, et al., “Endostatin and kidney fi brosis in aging: a case for antagonistic pleiotropy?” Am. J. Physiol. Heart Circ. Physiol., 306, No. 12, H1692–H1699 (2014), https://doi.org/10.1152/ajpheart.00064.2014.
G. Liszt, E. Ford, M. Kurtev, and L. Guarente, “Mouse Sir2 homolog SIRT6 is a nuclear ADP-ribosyltransferase,” J. Biol. Chem., 280, 21313–21320 (2005), https://doi.org/10.1074/jbc.M413296200.
L. Liu, C. Peritore, J. Ginsberg, et al., “Protective role of SIRT5 against motor defi cit and dopaminergic degeneration in MPTPinduced mice model of Parkinson’s disease,” Behav. Brain Res., 281, 215–221 (2015), https://doi.org/10.1016/j.bbr.2014.12.035.
D. B. Lombard and B. M. Zwaans, “SIRT3: as simple as it seems?” Gerontology, 60, No. 1, 56–64 (2014), https://doi.org/10.1159/000354382.
H. Luo, W. C. Mu, and R. Karki, et al., “Mitochondrial stress-initiated aberrant activation of the NLRP3 infl ammasome regulates the functional deterioration of hematopoietic stem cell aging,” Cell Rep., 26, 945–954 (2019), https://doi.org/10.1016/j.celrep.2018.12.101.
X. Y. Luo, S. L. Qu, and Z. H. Tang, et al., “SIRT1 in cardiovascular aging,” Clin. Chim. Acta, 437, 106–114 (2014), https://doi.org/10.1016/j.cca.2014.07.019.
Y. X. Luo, X. Tang, X. Z. An, et al., “SIRT4 accelerates Ang IIinduced pathological cardiac hypertrophy by inhibiting manganese superoxide dismutase activity,” Eur. Heart J., 38, No. 18, 1389–1398 (2017), https://doi.org/10.1093/eurheartj/ehw138.
S. Masri, P. Rigor, M. Cervantes, et al., “Partitioning circadian transcription by SIRT6 leads to segregated control of cellular metabolism,” Cell, 158, No. 3, 659–672 (2014), https://doi.org/10.1016/j.cell.2014.06.050.
E. McDonnell, B. S. Peterson, H. M. Bomze, and M. D. Hirschey, “SIRT3 regulates progression and development of diseases of aging,” Trends Endocrinol. Metab., 26, No. 9, 486–492 (2015), https://doi.org/10.1016/j.tem.2015.06.001.
Z. Min, J. Gao, and Y. Yu, “The roles of mitochondrial sirt4 in cellular metabolism,” Front. Endocrinol. (Lausanne), 9, 783 (2019), https://doi.org/10.3389/fendo.2018.00783.
M. Mohrin, J. Shin, Y. Liu, et al., “Stem cell aging. A mitochondrial UPR-mediated metabolic checkpoint regulates hematopoietic stem cell aging,” Science, 347, No. 6228, 1374–1377 (2015), https://doi.org/10.1126/science.aaa2361.
R. Mostoslavsky, K. F. Chua, D. B. Lombard, et al., “Genomic instability and aging-like phenotype in the absence of mammalian SIRT6,” Cell, 124, No. 2, 315–329 (2006), https://doi.org/10.1016/j.cell.2005.11.044.
M. C. Motta, N. Divecha, M. Lemieux, et al., “Mammalian SIRT1 represses forkhead transcription factors,” Cell, 116, 551–563 (2004), https://doi.org/10.1016/s0092-8674(04)00126-6.
R. Nogueiras, K. M. Habegger, N. Chaudhary, et al., “Sirtuin 1 and sirtuin 3: physiological modulators of metabolism,” Physiol. Rev., 92, No. 3, 1479–1514 (2012), https://doi.org/10.1152/physrev.00022.2011.
B. J. North, M. A. Rosenberg, and K. B. Jeganathan, et al., “SIRT2 induces the checkpoint kinase BubR1 to increase lifespan,” EMBO J., 33, No. 13, 1438–1453 (2014), 10.15252/embj.201386907.
C. O’Callaghan and A. Vassilopoulos, “Sirtuins at the crossroads of stemness, aging, and cancer,” Aging Cell, 16, No. 6, 1208–1218 (2017), https://doi.org/10.1111/acel.12685.
C. O’Callaghan, and A. Vassilopoulos, “Sirtuins at the crossroads of stemness, aging, and cancer,” Aging Cell, 16, No. 6, 1208–1218 (2017), https://doi.org/10.1111/acel.12685.
X. Ou, M. R. Lee, X. Huang, et al., “SIRT1 positively regulates autophagy and mitochondria function in embryonic Stem Cells under oxidative stress,” Stem Cells, 32, 1183–1194 (2014), https://doi.org/10.1002/stem.1641.
H. Pan, D. Guan, X. Liu, et al., “SIRT6 safeguards human mesenchymal stem cells from oxidative stress by coactivating NRF2,” Cell Res., 26, No. 2, 190–205 (2016), https://doi.org/10.1038/cr.2016.4.
S. Paredes, M. Angulo-Ibanez, L. Tasselli, et al., “The epigenetic regulator SIRT7 guards against mammalian cellular senescence induced by ribosomal DNA instability,” J. Biol. Chem., 293, No. 28, 11242–11250 (2018), https://doi.org/10.1074/jbc.AC118.003325.
S. Parik, S. Tewary, C. Ayyub, and U. Kolthur-Seetharam, “Loss of mitochondrial SIRT4 shortens lifespan and leads to a decline in physical activity,” J. Biosci, 43, No. 2, 243–247 (2018).
J. Park, Y. Chen, D. X. Tishkoff, et al., “SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways,” Mol. Cell., 50, 919–930 (2013), https://doi.org/10.1016/j.molcel.2013.06.001.
R. Paulin, P. Dromparis, G. Sutendra, et al., “Sirtuin 3 defi ciency is associated with inhibited mitochondrial function and pulmonary arterial hypertension in rodents and humans,” Cell Metab., 20, No. 5, 827–839 (2014), https://doi.org/10.1016/j.cmet.2014.08.011.
A. Salminen, K. Kaarniranta, and A. Kauppinen, “Crosstalk between oxidative stress and SIRT1: impact on the aging process,” Int. J. Mol. Sci., 14, No. 2, 3834–3859 (2013), https://doi.org/10.3390/ijms14023834.
A. Satoh, C. S. Brace, N. Rensing, et al., “SIRT1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH,” Cell Metab., 18, 416–430 (2013), https://doi.org/10.1016/j.cmet.2013.07.013.
J. Shih, L. Liu, A. Mason, et al., “Loss of SIRT4 decreases GLT-1- dependent glutamate uptake and increases sensitivity to kainic acid,” J. Neurochem., 131, No. 5, 573–581 (2014), https://doi.org/10.1111/jnc.12942.
D. M. Silberman, K. Ross, and P. H. Sande, et al., “SIRT6 is required for normal retinal function,” PLoS One, 9, No. 6, e98831 (2014), https://doi.org/10.1371/journal.pone.0098831.
W. Song, Y. Song, B. Kincaid, et al., “Mutant SOD1G93A triggers mitochondrial fragmentation in spinal cord motor neurons: neuroprotection by SIRT3 and PGC-1α,” Neurobiol. Dis., 51, 72–81 (2013), https://doi.org/10.1016/j.nbd.2012.07.004.
S. Sun, Z. Liu, Y. Feng, et al., “Sirt6 deacetylase activity regulates circadian rhythms via Per2,” Biochem. Biophys. Res. Commun, 511, No. 2, 234–238 (2019), https://doi.org/10.1016/j.bbrc.2019.01.143.
N. R. Sundaresan, M. Gupta, G. Kim, et al., “Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice,” J. Clin. Invest., 119, No. 9, 2758–2771 (2009), https://doi.org/10.1172/JCI39162.
B. Sung, J. W. Chung, H. R. Bae, et al., “Humulus japonicus extract exhibits antioxidative and anti-aging effects via modulation of the AMPK-SIRT1 pathway,” Exp. Ther. Med., 9, No. 5, 1819–1826 (2015), https://doi.org/10.3892/etm.2015.2302.
B. L. Tang, “Is SIRT6 Activity neuroprotective and how does it differ from SIRT1 in this regard?” Front. Cell. Neurosci., 11, 165 (2017), https://doi.org/10.3389/fncel.2017.00165.
Y. Tao, C. Huang, Y. Huang, et al., “SIRT4 suppresses inflammatory responses in human umbilical vein endothelial cells,” Cardiovasc. Toxicol, 15, No. 3, 217–223 (2015), https://doi.org/10.1007/s12012-014-9287-6.
J. Tian and L. Yuan, “Sirtuin 6 inhibits colon cancer progression by modulating PTEN/AKT signaling,” Biomed. Pharmacother., 106, 109–116 (2018), https://doi.org/10.1016/j.biopha.2018.06.070.
D. Tomaselli, C. Steegborn, A. Mai, and D. Rotili, “Sirt4: A multifaceted enzyme at the crossroads of mitochondrial metabolism and cancer,” Front. Oncol., 10, 474 (2020), https://doi.org/10.3389/fonc.2020.00474.
R. A. H. van de Ven, D. Santos, and M. C. Haigis, “Mitochondrial sirtuins and molecular mechanisms of aging,” Trends Mol. Med., 23, No. 4, 320–331 (2017), https://doi.org/10.1016/j.molmed.2017.02.005.
H. Vaziri, S. K. Dessain, E. Ng Eaton, et al., “HSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase,” Cell, 107, No. 2, 149–159 (2001), https://doi.org/10.1016/s0092-8674(01)00527-x.
N. Wang, Z. Luo, M. Jin, et al., “Exploration of age-related mitochondrial dysfunction and the anti-aging effects of resveratrol in zebrafish retina,” Aging (Albany NY), 11, No. 10, 3117–3137 (2019), 10.18632/aging.101966.
Y. Wang, J. Yang, T. Hong, et al., “SIRT2: Controversy and multiple roles in disease and physiology,” Ageing Res. Rev., 100961 (2019), https://doi.org/10.1016/j.arr.2019.100961.
J. G. Wood, B. Schwer, P. C. Wickremesinghe, et al., “Sirt4 is a mitochondrial regulator of metabolism and lifespan in Drosophila melanogaster,” Proc. Natl. Acad. Sci. USA, 115, No. 7, 1564–1569 (2018), https://doi.org/10.1093/geroni/igy023.345.
J. J. Wu, J. Liu, E. B. Chen, et al., “Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression,” Cell Rep., 4, 913–920 (2013), https://doi.org/10.1016/j.celrep.2013.07.030.
D. Xu, X. Jiang, H. He, et al., “SIRT2 functions in aging, autophagy, and apoptosis in post-maturation bovine oocytes,” Life Sci., 232, 116639 (2019), https://doi.org/10.1016/j.lfs.2019.116639.
S. Yamamura, Y. Izumiya, S. Araki, et al., “Cardiomyocyte Sirt (Sirtuin) 7 ameliorates stress-induced cardiac hypertrophy by interacting with and deacetylating GATA4,” Hypertension, 75, No. 1, 98–108 (2020), https://doi.org/10.1161/HYPERTENSIONAHA.119.13357.
B. Yang, X. Fu, L. Shao, et al., “Aberrant expression of SIRT3 is conversely correlated with the progression and prognosis of human gastric cancer,” Biochem. Biophys. Res. Commun, 443, No. 1, 156– 160 (2014), https://doi.org/10.1016/j.bbrc.2013.11.068.
M. Yang, Y. Peng, W. Liu, et al., “Sirtuin 2 expression suppresses oxidative stress and senescence of nucleus pulposus cells through inhibition of the p53/p21 pathway,” Biochem. Biophys. Res. Commun, 513, No. 3, 616–622 (2019), https://doi.org/10.1016/j.bbrc.2019.03.200.
F. Yeung, J. E. Hoberg, C. S. Ramsey, et al., “Modulation of NFkappa B-dependent transcription and cell survival by the SIRT1 deacetylase,” EMBO J., 23, No. 12, 2369–2380 (2004), https://doi.org/10.1038/sj.emboj.7600244.
W. You, D. Rotili, T. M. Li, et al., “Structural basis of sirtuin 6 activation by synthetic small molecules,” Angew. Chem. Int. Ed. Engl., 56, No. 4, 1007–1011 (2017), https://doi.org/10.1002/anie.201610082.
M. J. Zarzuelo, R. López-Sepúlveda, M. Sánchez, et al., “SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: implications for vascular aging,” Biochem. Pharmacol., 85, No. 9, 1288–1296 (2013), https://doi.org/10.1016/j.bcp.2013.02.015.
L. Zeng, Y. Yang, and Y. Hu, et al., “Age-related decrease in the mitochondrial sirtuin deacetylase Sirt3 expression associated with ROS accumulation in the auditory cortex of the mimetic aging rat model,” PLoS One, 9, No. 2, e88019 (2014), https://doi.org/10.1371/journal.pone.0088019.
C. Z. Zhang, L. Liu, M. Cai, et al., “Low SIRT3 expression correlates with poor differentiation and unfavorable prognosis in primary hepatocellular carcinoma,” PLoS One, 7, No. 12, e51703 (2012), https://doi.org/10.1371/journal.pone.0051703.
H. Zhang, X. Yang, and X. Pang, et al., “Genistein protects against ox- LDL-induced senescence through enhancing SIRT1/LKB1/AMPKmediated autophagy flux in HUVECs,” Mol. Cell Biochem., 455, No. 1–2, 127–134 (2019), https://doi.org/10.1007/s11010-018-3476-8.
Y. Zhang, S. L. Mi, and N. Hu, et al., “Mitochondrial aldehyde dehydrogenase 2 accentuates aging-induced cardiac remodeling and contractile dysfunction: role of AMPK, SIRT1, and mitochondrial function,” Free Radic. Biol. Med., 71, 208–220 (2014), https://doi.org/10.1016/j.freeradbiomed.2014.03.018.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Uspekhi Fiziologicheskikh Nauk, Vol. 53, No. 1, pp. 16–27, January–March, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pukhalskaia, A.E., Kvetnoy, I.M., Linkova, N.S. et al. Sirtuins and Aging. Neurosci Behav Physi 52, 1482–1490 (2022). https://doi.org/10.1007/s11055-023-01379-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-023-01379-8