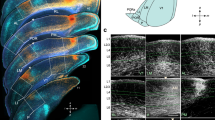
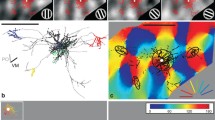
The direct neuronal connections of the cortical visual areas at the lower level of the hierarchy with the areas of subsequent levels were studied by microiontophoretic administration of horseradish peroxidase into individual vertical columns in areas 19 and 21a and the distribution of retrograde labeled neurons in areas 17 and 18 was analyzed in cats. Columns in areas 19 and 21a, as compared with the eye-dominant columns of area 18, receive additional cortical inputs from the ipsilateral transition zone 17/18, which indicates a more complex structure of neuronal connections. The locations of input neurons from the transition zones 17/18 of different hemispheres were found to be mirror symmetrical, such that neurons in areas 19 and 21a columns can be tuned to loci located in the central sagittal plane of three-dimensional space. Inputs from areas 17 and 18 of the ipsilateral hemisphere, located outside the transition zone 17/18, were also identified, and these, in combination with inputs from both transition zones, can enable neurons in areas 19 and 21a columns to encode more complex stereo-features of objects.
Similar content being viewed by others
References
Alekseenko, S. V. and Shkorbatova, P. Yu., “Microstructure of intrahemispheric bottom-up pathways in the extrastriate areas of the cat cortex,” Integr. Fiziol., 2, No. 2, 205–214 (2021).
Alekseenko, S. V., Toporova, S. N., and Makarov, F. N., “Neuronal connections uniting the visual hemifields,” Sens. Sistemy, 16, No. 2, 83–88 (2002).
Alekseenko, S. V., Toporova, S. N., and Makarov, F. N., “Neuronal connections of the cortex and reconstruction of the visual space,” Neurosci. Behav. Physiol., 35, No. 4, 435–442 (2005).
Bakin, J. S., Nakayama, K., and Gilbert, C. D., “Visual responses in monkey areas V1 and V2 to three-dimensional surface confi gurations,” J. Neurosci., 20, No. 21, 8188–8198 (2000).
Barlow, H. B., Blakemore, C., and Pettigrew, J. D., “The neural mechanisms of binocular depth discrimination,” J. Physiol., 193, No. 2, 327–342 (1967).
Berman, N., Payne, B. R., Labar, D. R., and Murphy, E. H., “Functional organization of neurons in cat striate cortex: variations in ocular dominance and receptive-field type with cortical laminae and location in visual field,” J. Neurophysiol., 48, 1362–1377 (1982).
Cumming, B. G. and Parker, A. J., “Binocular neurons in V1 of awake monkeys are selective for absolute, not relative, disparity,” J. Neurosci., 19, 5602–5618 (1999).
Dreher, B., “Thalamocortical and corticocortical interconnections in the cat visual system: relation to the mechanisms of information processing,” in: Visual Neuroscience, Pettigrew, J. D. et al. (eds.), Cambridge University Press, London (1985), pp. 290–314.
Duysens, J., Orban, G. A., van der Glas, H. W., and de Zegher, F. E., “Functional properties of area 19 as compared to area 17 of the cat,” Brain Res., 231, 279–291 (1982).
Felleman, D. J. and Van Essen, D. C., “Distributed hierarchical processing in the primate Cerebral Cortex,” Cereb. Cortex, 1, 1–47 (1991).
Guillemot, J.-P., Paradis, M.-C., Samson, A., et al., “Binocular interaction and disparity coding in area 19 of visual cortex in normal and split-chiasm cats,” Exp. Brain Res., 94, 405–417 (1993).
Harutiunian-Kozak, B. A., Grigorian, G. G., Kozak, J. A., et al., “Orientation sensitive properties of visually driven neurons in extrastriate area 21a of cat cortex,” Arch. Ital. Biol., 146, No. 2, 119–130 (2008).
Houzel, J. C., Milleret, C., and Innocenti, G., “Morphology of callosal axons interconnecting areas 17 and 18 of the cat,” Eur. J. Neurosci., 6, No. 6, 898–917 (1994).
Hubel, D. H. and Wiesel, T. N., Brain and Visual Perception, Oxford University Press, New York, Oxford (2005).
Innocenti, G. M., “Network causality, axonal computations, and Poffenberger,” Exp. Brain Res., 235, 2349–2357 (2017).
Ivanov, R. S., Bondar’, I. V., Saltykov, K. A., and Shevelev, I. A., “The area of activation zones in field 17 of the cat brain on presentation of grids with different orientations,” Zh. Vyssh. Nerv. Deyat., 56, No. 4, 516–522 (2006).
Khayat, P. S., Saint-Amour, D., Molotchnikoff, S., et al., “Cellular response to texture and form defined by motion in area 19 of the cat,” Eur. J. Neurosci., 5, 1727–1738 (2000).
Kim, T., Bair, W., and Pasupathy, A., “Neural coding for shape and texture in macaque area V4,” J. Neurosci., 39, No. 24, 4760–4774 (2019).
Li, Y., Zhang, C., Hou, C., et al., “Stereoscopic processing of crossed and uncrossed disparities in the human visual cortex,” BMC Neurosci., 18, No. 1, 80 (2017), https://doi.org/10.1186/s12868-017-0395-7.
Li, Z. and Shigemasu, H., “Generalized representation of stereoscopic surface shape and orientation in the human visual cortex,” Front. Hum. Neurosci., 13, 283; eCollection 2019 (2019), https://doi.org/10.3389/fnhum.2019.00283.
Lu, Y., Yin, J., Chen, Z., et al., “Revealing detail along the visual hierarchy: neural clustering preserves acuity from V1 to V4,” Neuron, 98, No. 2, 417–428 (2018).
Olavarría, J. F., “Callosal connections correlate preferentially with ipsilateral cortical domains in cat areas 17 and 18, and with contralateral domains in the 17/18 transition zone,” J. Comp. Neurol., 433, 441–457 (2001).
Parker, A. J., “Intermediate level cortical areas and the multiple roles of area V4,” Curr. Opin. Physiol., 16, 61–66 (2020).
Parker, A. J., Smith, J. E. T., and Krug, K., “Neural architectures for stereo vision,” Phil. Trans. R. Soc. B., 371, 20150261 (2016).
Pasupathy, A., Popovkina, D. V., and Kim, T., “Visual functions of primate area V4,” Annu. Rev. Vis. Sci., (2020), https://doi.org/10.1146/annurevvision-030320-041306.
Payne, B. R., “Representation of the ipsilateral visual fi eld in the transition zone between areas 17 and 18 of the cat’s cerebral cortex,” Vis. Neurosci., 4, No. 5, 445–474 (1990).
Pettine, W. W., Steinmetz, N. A., and Moore, T., “Laminar segregation of sensory coding and behavioral readout in macaque V4,” Proc. Natl. Acad. Sci. USA, 116, 14,749–14,754 (2019).
Ramachandra, V., Pawlak, V., Wallace, D. J., and Kerr, J. N. D., “Impact of visual callosal pathway is dependent upon ipsilateral thalamus,” Nat. Commun., 11, No. 1, 1889 (2020), https://doi.org/10.1038/s41467-020-15672-4.
Rochefort, N. L., Buzas, P., Kisvarday, Z. F., et al., “Layout of transcallosal activity in cat visual cortex revealed by optical imaging,” NeuroImage, 36, 804–821 (2007).
Rockland, K. S., “Axon collaterals and brain states,” Front. Syst. Neurosci., 12, 32, eCollection 2018 (2018), https://doi.org/10.3389/fnsys.2018.00032.
Salin, P. A., Girard, P., Kennedy, H., and Bullier, J., “Visuotopic organization of corticocortical connections in the visual system of the cat,” J. Comp. Neurol., 320, No. 4, 415–434 (1992).
Scannell, J. W., Blakemore, C., and Young, M. P., “Analysis of connectivity in the cat cerebral cortex,” J. Neurosci., 15, No. 2, 1463–1483 (1995).
Thomas, O. M., Cumming, B. G., and Parker, A. J., “A specialization for relative disparity in V2,” Nature Neurosci., 5, 472–478 (2002).
Toporova, S. N., Alekseenko, S. V., and Makarov, F. N., “The spatial distribution of horizontal connections in area 18 of the cortex in cats,” Neurosci. Behav. Physiol., 31, No. 4, 345–348 (2001).
Tusa, R. J., Palmer, L. A., and Rosenquist, A. C., “Multiple cortical visual areas: Visual fi eld topography in the cat,” in: Cortical Sensory Organization, Humana Press, New York (1981), Vol. 2, pp. 1–31.
Villeneuve, M. Y., Vanni, M. P., and Casanova, C., “Modular organization in area 21a of the cat revealed by optical imaging: comparison with the primary visual cortex,” Neuroscience, 164, No. 3, 1320–33 (2009).
Wimborne, B. M. and Henry, G. H., “Response characteristics of the cells in the cortical area 21a of the cat with special reference to orientation specificity,” J. Physiol., 449, 457–478 (1992).
Wunderle, T., Eriksson, D., Peiker, C., and Schmid, K. E., “Input and output gain modulation by the lateral interhemispheric network in early visual cortex,” J. Neurosci., 35, No. 20, 7682–7694 (2015).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Vysshei Nervnoi Deyatel’nosti imeni I. P. Pavlova, Vol. 72, No. 2, pp. 250–258, March–April, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alekseenko, S.V., Shkorbatova, P.Y. Microstructure of Neuronal Connections between the Visual Areas of the Cortex at Different Hierarchical Levels. Neurosci Behav Physi 52, 1270–1276 (2022). https://doi.org/10.1007/s11055-023-01356-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-023-01356-1