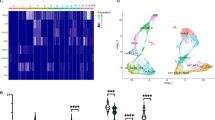
Aberrant synchronous β oscillations in motor neural networks in dopamine (DA) deficiency are associated with motor impairments in Parkinson’s disease (PD). The sources and mechanisms of their development are unclear. The aim of the present work was to determine the role of the external part of the globus pallidus (GPe) and the central component of the basal ganglia (BG) in generating and transmitting β oscillations in motor neural networks in a model of PD in rats. Analysis of local field potentials (LFP) in traces from the motor area of the cerebral cortex (MCx) and BG nuclei revealed the greatest β-oscillation (30–36 Hz) power and coherence levels in the MCx and reticular part of the substantia nigra (SNr) of hemispheres with DA deficit, while their levels in the dorsal segment of the lateral striatum (dStr) and GPe and coherence with the MCx and Snr were significantly lower. Apart from β oscillations, increases in the coherence of γ oscillations in the range 50–56 Hz were observed exclusively in the dStr and GPe in DA deficiency and their transient appearance in control rats coincided with the onset of difficulties with walking. Stimulation of DA receptors with levodopa decreased synchronization in the neural networks of hemispheres with DA deficiency and restored normal locomotion. Differences between the two types of activity (β and γ oscillations) in traces from the GPe in DA deficiency provide evidence of the complexity of the organization of motor neural networks which in normal conditions control different aspects of locomotion.
Similar content being viewed by others
References
Abdi, A., Mallet, N., Mohamed, F. Y., et al., “Prototypic and arkypallidal neurons in the dopamine-intact external globus pallidus,” J. Neurosci., 17, 6667–6688 (2015).
Abecassis, Z. A., Berceau, B. L., Win, P. H., et al., “Npas1+–Nkx2.1+ neurons are an integral part of the cortico-pallido-cortical loop,” J. Neurosci., 4, 743–768 (2020).
Abrahao, K. P. and Lovinger, D. M., “Classification of GABAergic neuron subtypes from the globus pallidus using wild-type and transgenic mice,” J. Physiol., 596, 4219–4235 (2018).
Albin, R. L., Young, A. B., and Penney, J. B., “The functional anatomy of basal ganglia disorders,” Trends Neurosci., 12, 366–375 (1989).
Avila, I., Parr-Brownlie, L. C., Brazhnik, et al., “Beta frequency synchronization in basal ganglia output during rest and walk in a hemiparkinsonian rat,” Exp. Neurol., 2, 307–319 (2010).
Baaske, M. K., Kormann, E., Holt, A. B., et al., “Parkinson’s disease uncovers an underlying sensitivity of subthalamic nucleus neurons to beta-frequency cortical input in vivo,” Neurobiol. Dis., 146, 105– 119 (2020).
Belluscio, V., Stuart, S., Bergamini, E., et al., “The association between prefrontal cortex activity and turning behavior in people with and without freezing of gait,” Neuroscience, 416, 168–176 (2019).
Berke, J. D., “Fast oscillations in cortical-striatal networks switch frequency following rewarding events and stimulant drugs,” Eur. J. Neurosci., 30, 848–859 (2009).
Bevan, M. D., Magill, P. J., Terman, D., et al., “Review. Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network,” Trends Neurosci., 10, 525–531 (2002).
Brazhnik, E., Cruz, A. V., Avila, I., et al., “State-dependent spike and local fi eld synchronization between motor cortex and substantia nigra in hemiparkinsonian rats,” J. Neurosci., 32, 7869–7880 (2012).
Brazhnik, E., McCoy, A. J., Novikov, N., et al., “Ventral medial thalamic nucleus promotes synchronization of increased high beta oscillatory activity in the basal ganglia-thalamocortical Network of the hemiparkinsonian rat,” J. Neurosci., 36, 4196–4208 (2016).
Brittain, J. S., Sharott, A., and Brown, P., “The highs and lows of beta activity in cortico-basal ganglia loops,” Eur. J. Neurosci., 39, 1951– 1959 (2014).
Brown, P., “Bad oscillations in Parkinson’s disease,” J. Neural. Transm., 70, 27–30 (2006).
Cacciola, A., Milardi, D., Bertino, S., et al., “Structural connectivity-based topography of the human globus pallidus: Implications for therapeutic targeting in movement disorders,” Mov. Disord., 7, 987–996 (2019).
Cardin, J. A., Carlén, M., Meletis, K., et al., “Driving fast-spiking cells induces gamma rhythm and controls sensory responses,” Nature, 459, 663–667 (2009).
Chen, M. C., Ferrari, L., Sacchet, M. D., et al., “Identification of a direct GABAergic pallidocortical pathway in rodents,” Eur. J. Neurosci. Mov. Disord., 30, 293–295 (2015).
Choi, K., Holly, E., Davatolhagh, M. F., et al., “Integrated anatomical and physiological mapping of striatal afferent projections,” Eur. J. Neurosci., 49, No. 5, 623–636 (2019).
Chuhma, N., “Functional connectome analysis of the striatum with optogenetics,” Adv. Exp. Med. Biol., 1293, 417–428 (2021).
Corbit, V. L., Whalen, T. C., Zitelli, K. T., et al., “Pallidostriatal projections promote β oscillations in a dopamine-depleted biophysical network model,” J. Neurosci., 2, 5556–5571 (2016).
de la Crompe, B., Aristieta, A., Leblois, A., et al., “The globus pallidus orchestrates abnormal network dynamics in a model of Parkinsonism,” Nat. Commun., 11, 1570–1584 (2020).
Dejean, C. A., Le Moine, C., Bioulac, B., et al., “Evolution of the dynamic properties of the cortex-basal ganglia network after dopaminergic depletion in rats,” Neurobiol. Dis., 2, 402–413 (2012).
Delaville, C., McCoy, A. J., Gerber, C. M., et al., “Subthalamic nucleus activity in the awake hemiparkinsonian rat: relationships with motor and cognitive networks,” J. Neurosci., 17, 6918–6930 (2015).
Dodson, P. D., Larvin, J. T., Duffell, J. M., et al., “Distinct developmental origins manifest in the specialized encoding of movement by adult neurons of the external globus pallidus,” Neuron, 86, 501–513 (2015).
Eid, L. and Parent, M., “Morphological evidence for dopamine interactions with pallidal neurons in primates,” Front. Neuroanat., 9, 1–14 (2015).
Gauthier, J., Parent, M., Levesque, M., and Parent, A., “The axonal arborization of single nigrostriatal neurons in rats,” Brain Res., 834, 228– 232 (1999).
Gittis, A. H., Berke, J. D., Bevan, M. D., et al., “New roles for the external globus pallidus in basal ganglia circuits and behavior,” J. Neurosci., 34, 15178–15183 (2014).
Glajch, K. E., Kelver, D. A., Hegeman, D. J., et al., “Npas1+ pallidal neurons target striatal projection neurons,” J. Neurosci., 36, 5472–5488 (2016).
Grewal, S. S., Holanda, V. M., and Middlebrook, E. Y., “Corticopallidal connectome of the globus pallidus externus in humans: An exploratory study of structural connectivity using probabilistic diffusion tractography,” Am. J. Neuroradiology, 11, 2120–2125 (2018).
Hegeman, D. J., Hong, E. S., Hernández, V. M., and Chan, C., “The external globus pallidus: progress and perspectives,” Eur. J. Neurosci., 10, 1239–1265 (2016).
Hernández, V. M., Hegeman, D. J., Cui, Q., et al., “Parvalbumin+ neurons and Npas1+ neurons are distinct neuron classes in the mouse external globus pallidus,” J. Neurosci., 35, 11,830–11,847 (2015).
Karube, F., Takahashi, S., Kobayashi, K., and Fujiyama, F., “Motor cortex can directly drive the globus pallidus neurons in a projection neuron type-dependent manner in the rat,” eLife, 8, e49511 (2019).
Ketzef, M. and Silberberg, G., “Differential synaptic input to external globus pallidus neuronal subpopulations in vivo,” Neuron, 109, No. 3, 516–529 (2021).
Kita, H., Globus pallidus external segment,” Prog. Brain Res., 160, 111–133 (2007).
Koelman, L. A. and Lowery, M. M., “Beta-band resonance and intrinsic oscillations in a biophysically detailed model of the subthalamic nucleus- globus pallidus network,” Front. Comput. Neurosci., 13, 1–24 (2019).
Lemaire, N., Hernández, L. F., Hu, D., et al., “Effects of dopamine depletion on LFP oscillations in striatum are task- and learning-dependent and selectively reversed by L-DOPA,” Proc. Natl. Acad. Sci. USA, 109, 18,126–18,131 (2012).
Litvak, V., Jha, A., Eusebio, A., et al., “Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson’s disease,” Brain, 134, 359–374 (2011).
Maidan, I., Bernad-Elazari, H., Gazit, E., et al., “Changes in oxygenated hemoglobin link freezing of gait to frontal activation in patients with Parkinson disease: an fNIRS study of transient motor-cognitive failures,” J. Neurol., 4, 899–908 (2015).
Mallet, N., Delgado, L., Chazalon, M., et al., “Cellular and synaptic dysfunctions in parkinson’s disease: Stepping out of the striatum,” Cells, 9, 1005 (2019).
Mallet, N., Micklem, B. R., Henny, P., et al., “Dichotomous organization of the external globus pallidus,” Neuron, 74, 1075–1086 (2012).
Mallet, N., Pogosyan, A., Marton, L. F., et al., “Parkinsonian beta oscillations in the external globus pallidus and their relationship with subthalamic nucleus activity,” J. Neurosci., 52, 14,245–14,258 (2008).
Mallet, N., Schmidt, R., Leventhal, D., et al., “Arkypallidal cells send a stop signal to striatum,” Neuron, 89, 308–316 (2016).
Mastro, K. J., Bouchard, R. S., Holt, H. A., and Gittis, A. H., “Transgenic mouse lines subdivide external segment of the globus pallidus (GPe) neurons and reveal distinct GPe output pathways,” J. Neurosci., 34, 2087–2099 (2014).
Mastro, K. J., Zitelli, K. T., Willard, A. M., et al., “Cell-specifi c pallidal intervention induces long-lasting motor recovery in dopamine-depleted mice,” Nat. Neurosci., 20, 815–823 (2017).
McCarthy, M. M., Moore-Kochlacs, C., Gu, X., et al., “Striatal origin of the pathologic beta oscillations in Parkinson’s disease,” Proc. Natl. Acad. Sci. USA, 108, 11,620–11,625 (2011).
McGregor, M. M., McKinsey, G. L., Girasole, A. E., et al., “Functionally distinct connectivity of developmentally targeted striosome neurons,” Cell Rep., 29, No. 6, 1419–1428 (2019).
Nambu, A., Tokuno, H., and Takada, M., “Functional signifi cance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway,” Neurosci. Res., 2,111–117 (2002).
Neumann, W. J., Degen, K., Schneider, G. H., et al., “Subthalamic synchronized oscillatory activity correlates with motor impairment in patients with Parkinson’s disease,” Mov. Disord., 31,1748–1751 (2016).
Nevado-Holgado, A. J., Mallet, N., Magill, P. J., and Bogacz, R., “Effective connectivity of the subthalamic nucleus–globus pallidus network during Parkinsonian oscillations,” J. Physiol., 592, No. 7, 1429– 1455 (2014).
Pamukcu, A., Cui, Q., Xenias, H. S., et al., “Parvalbumin (+) and Npas1(+) pallidal neurons have distinct circuit topology and function,” J. Neurosci., 40, 7855–7876 (2020).
Parent, A. and Hazrati, L. N., “Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop,” Brain Res. Brain Res. Rev., 1, 91–127 (1995).
Plenz, D. and Kital, S. T., “A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus,” Nature, No. 6745, 677–682 (1999).
Pogosyan, A., Yoshida, F., Chen, C. C., et al., “Parkinsonian impairment correlates with spatially extensive subthalamic oscillatory synchronization,” Neuroscience, 171, No. 1, 245–257 (2010).
Polyakova, Z., Chiken, S., Hatanaka, N., and Nambu, A., “Cortical control of subthalamic neuronal activity through the hyperdirect and indirect pathways in monkeys,” J. Neurosci., 39, 7451–7463 (2020).
Rodriguez-Sabate, C., Morales, I., Monton, F., and Rodriguez, M., “The influence of Parkinson’s disease on the functional connectivity of the motor loop of human basal ganglia,” Parkinsonism Relat. Disord., 63, 100–105 (2019).
Saunders, A., Oldenburg, I. A., Berezovskii, V. K., et al., “A direct GABAergic output from the basal ganglia to frontal cortex,” Nature, 521, 85–89 (2015).
Sharott, A., Gulberti, A., Hamel, W., et al., “Spatio-temporal dynamics of cortical drive to human subthalamic nucleus neurons in Parkinson’s disease,” Neurobiol. Dis., 112, 49–62 (2018).
Sharott, A., Gulberti, A., Zittel, S., et al., “Activity parameters of subthalamic nucleus neurons selectively predict motor symptom severity in Parkinson’s disease,” J. Neurosci., 34, 6273–6285 (2014).
Sharott, A., Magill, P. J., Harnack, D., et al., “Dopamine depletion increases the power and coherence of beta-oscillations in the cerebral cortex and subthalamic nucleus of the awake rat,” Eur. J. Neurosci., 5, 1413–1422 (2005).
Singh, A., “Oscillatory activity in the cortico-basal ganglia-thalamic neural circuits in Parkinson’s disease,” Eur. J. Neurosci., 8, 2869–2878 (2018).
Smith, J. B., Klug, J. R., Ross, D. L., et al., “Genetic-based dissection unveils the inputs and outputs of striatal patch and matrix compartments,” Neuron, 91, No. 5, 1069–1084. (2016).
Smith, Y. and Villalba, R., “Striatal and extrastriatal dopamine in the basal ganglia: an overview of its anatomical organization in normal and Parkinsonian brains,” Mov. Disord., 3, S534–547 (2008).
Tachibana, Y., Iwamuro, H., Kita, H., et al., “Subthalamo-pallidal interactions underlying parkinsonian neuronal oscillations in the primate basal ganglia,” Eur. J. Neurosci., 34, 1470–1484 (2011).
Weinberger, M., Hutchison, W. D., and Dostrovsky, J. O., “Pathological subthalamic nucleus oscillations in PD: can they be the cause of bradykinesia and akinesia?” Exp. Neurol., 1, 58–61 (2009).
West, T. O., Berthouze, L., Halliday, D. M., et al., “Propagation of beta/ gamma rhythms in the cortico-basal ganglia circuits of the parkinsonian rat,” J. Neurophysiol., 5, 1608–1628 (2018).
Yasukawa, T., Kita, T., Xue, Y., and Kita, H., “Rat intralaminar thalamic nuclei projections to the globus pallidus: a biotinylated dextran amine anterograde tracing study,” J. Comp. Neurol., 2, 153–167 (2004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Vysshei Nervnoi Deyatel’nosti imeni I. P. Pavlova, Vol. 72, No. 1, pp. 100–116, January–February, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Morozova, M.V., Brazhnik, E.S., Mysin, I.E. et al. The Contribution of the External Globus Pallidus to Basal Ganglia Circuit Oscillatory Activity in an Experimental Model of Parkinson’s Disease. Neurosci Behav Physi 52, 1061–1072 (2022). https://doi.org/10.1007/s11055-022-01334-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-022-01334-z