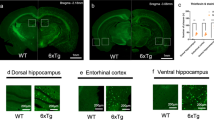
Alzheimer’s disease is the most widespread neurodegenerative disease in the world and produces significant adverse social-economic consequences. Despite numerous studies of the pathogenesis of this disease, its neuropathological mechanisms remain incompletely understood and current treatment methods lack sufficient efficacy. In recent decades, genetic models of Alzheimer’s disease in rodents have been used for detailed study of the molecular mechanisms of the development of this disease, this aspect being of decisive importance for its early diagnosis and effective therapy. Given the significant influence of β-amyloid on the manifestations of impairments in behavior and the development of cognitive dysfunction, this review presents the detailed characteristics of the 5xFAD transgenic mouse model as a valuable and necessary tool for studying the basic mechanisms underlying the cognitive and mental disorders in AD and their interaction with the cerebral microenvironment.
Similar content being viewed by others
References
Abe, Y., Ikegawa, N., Yoshida, K., et al., “Behavioral and electrophysiological evidence for a neuroprotective role of aquaporin-4 in the 5xFAD transgenic mice model,” Acta Neuropathol. Commun., 8, No. 1, 67 (2020).
Adams, S. J., Crook, R. J. P., Deture, M., et al., “Overexpression of wildtype murine tau results in progressive tauopathy and neurodegeneration,” Am. J. Pathol., 175, No. 4, 1598–1609 (2009).
Ahlemeyer, B., Halupczok, S., Rodenberg-Frank, E., et al., “Endogenous Murine Amyloid-β peptide assembles into aggregates in the aged C57BL/6J mouse suggesting these animals as a model to study pathogenesis of amyloid-β plaque formation,” J. Alzheimers Dis., 61, No. 4, 1425–1450 (2018).
Arrozi, A. P., Shukri, S. N. S., Ngah, W. Z. W., et al., “Evaluation of the expression of amyloid precursor protein and the ratio of secreted amyloid beta 42 to amyloid beta 40 in SH-SY5Y cells stably transfected with wild-type, single-mutant and double-mutant forms of the APP gene for the Study of Alzheimer’s disease pathology,” Appl. Biochem. Biotechnol., 183, No. 3, 853–866 (2017).
Babulal, G. M., Chen, S., Williams, M. M., et al., “Depression and Alzheimer’s Disease biomarkers predict driving decline,” J. Alzheimers Dis., 66, No. 3, 1213–1221 (2018).
Bagyinszky, E., Park, S. A., Kim, H. J., et al., “PSEN1 L226F mutation in a patient with early-onset Alzheimer’s disease in Korea,” Clin. Interv. Aging, 11, 1433–1440 (2016).
Bories, C., Guitton, M. J., Julien, C., et al., “Sex-dependent alterations in social behaviour and cortical synaptic activity coincide at different ages in a model of Alzheimer’s disease,” PLoS One, 7, No. 9, 1–8 (2012).
Caldwell, A. B., Liu, Q., Schroth, G. P., et al., “Dedifferentiation and neuronal repression define familial Alzheimer’s disease,” Sci. Adv., 6, No. 46, eaba5933 (2020).
Canevelli, M., Piscopo, P., Talarico, G., et al., “Familial Alzheimer’s disease sustained by presenilin 2 mutations: systematic review of literature and genotype–phenotype correlation,” Neurosci. Biobehav.Rev., 42, 170–179 (2014).
Cao, Q., Wang, W., Williams, J. B., et al., “Targeting histone K4 trimethylation for treatment of cognitive and synaptic deficits in mouse models of Alzheimer’s disease,” Sci. Adv., 6, No. 50, eabc8096 (2020).
Chishti, M. A., Yang, D. S., Janus, C., et al., “Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695,” J. Biol. Chem., 276, No. 24, 21562–21570 (2001).
Creighton, S. D., Mendell, A. L., Palmer, D., et al., “Dissociable cognitive impairments in two strains of transgenic Alzheimer’s disease mice revealed by a battery of object-based tests,” Sci. Rep., 9, No. 1, 57 (2019).
Cruchaga, C., Del-Aguila, J. L., Saef, B., et al., “Polygenic risk score of sporadic late-onset Alzheimer’s disease reveals a shared architecture with the familial and early-onset forms,” Alzheimers Dement., 14, No. 2, 205–214 (2018).
Cryan, J. F., Mombereau, C., and Vassout, A., “The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice,” Neurosci. Biobehav. Rev., 29, No. 4–5, 571–625 (2005).
Cummings, J., Ritter, A., and Rothenberg, K., “Advances in management of neuropsychiatric syndromes in neurodegenerative diseases,” Curr. Psychiatry Rep., 21, No. 8, 79 (2019).
D’Argenio, V. and Sarnataro, D., “New Insights into the molecular bases of familial alzheimer’s disease,” J. Pers. Med., 10, No. 2, 26 (2020).
DeBay, D. R., Reid, G. A., Macdonald, I. R., et al., “Butyrylcholinesteraseknockout reduces fibrillar β-amyloid and conserves 18FDG retention in 5XFAD mouse model of Alzheimer’s disease,” Brain Res., 1671, 102–110 (2017).
Devi, L. and Ohno, M., “A combination Alzheimer’s therapy targeting BACE1 and neprilysin in 5XFAD transgenic mice,” Mol. Brain, 8, 19 (2015).
Devi, L. and Ohno, M., “Mechanisms that lessen benefits of β-secretase reduction in a mouse model of Alzheimer’s disease,” Transl. Psychiatry, 3, No. 7, e284 (2013).
Devi, L. and Ohno, M., “Phospho-eIF2α level is important for determining abilities of BACE1 reduction to rescue cholinergic neurodegeneration and memory defects in 5XFAD mice,” PLoS One, 5, No. 9, e12974 (2010).
Diekelmann, S. and Born, J., “The memory function of sleep,” Nat. Rev. Neurosci., 11, No. 2, 114–126 (2010).
Eimer, W. A. and Vassar, R., “Neuron loss in the 5XFAD mouse model of Alzheimer’s disease correlates with intraneuronal Aβ42 accumulation and caspase-3 activation,” Mol. Neurodegener., 8, 2 (2013).
Elder, G. A., Sosa, M. A. G., and Gasperi, R. D., “Transgenic mouse models of Alzheimer’s disease,” Mt. Sinai J. Med., 77, No. 1, 69–81 (2010).
Filali, M., Lalonde, R., and Rivest, S., “Cognitive and non-cognitive behaviors in an APPswe/PS1 bigenic model of Alzheimer’s disease,” Genes Brain Behav., 8, No. 2, 143–148 (2009).
Fjell, A. M. and Walhovd, K. B., “Structural brain changes in aging: courses, causes and cognitive consequences,” Rev. Neurosci., 21, No. 3, 187–221 (2010).
Flanigan, T. J., Xue, Y., Rao, S. K., et al., “Abnormal vibrissa-related behavior and loss of barrel field inhibitory neurons in 5xFAD transgenics,” Genes Brain Behav., 13, No. 5, 488–500 (2014).
Forrest, S. L., Kril, J. J., Stevens, C. H., et al., “Retiring the term FTDP-17 as MAPT mutations are genetic forms of sporadic frontotemporal tauopathies,” Brain, 141, No. 2, 521–534 (2018).
Garre-Olmo, J., “Epidemiology of Alzheimer’s disease and other dementias,” Rev. Neurol., 66, No. 11, 377–386 (2018).
Gerakis, Y. and Hetz, C., “Brain organoids: a next step for humanized Alzheimer’s disease models?” Mol. Psychiatry, 24, No. 4, 474–478 (2019).
Giannoni, P., Arango-Lievano, M., Neves, I. D., et al., “Cerebrovascular pathology during the progression of experimental Alzheimer’s disease,” Neurobiol. Dis., 88, 107–117 (2016).
Giau, V. V., Bagyinszky, E., Youn, Y. C., et al., “APP, PSEN1, and PSEN2 mutations in Asian patients with early-onset Alzheimer disease,” Int. J. Mol. Sci., 20, No. 19, 4757 (2019).
Goate, A., “Segregation of a missense mutation in the amyloid beta-protein precursor gene with familial Alzheimer’s disease,” J. Alzheimers Dis., 9, 341–347 (2006).
Gorina, Y. V., Komleva, Yu. K., Lopatina, O. L., et al., “Effects of insulin resistance on impairments to glucose metabolism in the amygdala of the brain in experimental Alzheimer’s disease,” Byull. Sibirsk. Med., 6, 1–5 (2017a).
Gorina, Y. V., Komleva, Yu. K., Lopatina, O. L., et al., “Behavioral phenotypic analysis of animals with a genetic model of Alzheimer’s disease,” Biomeditsina, 3, 47–59 (2017b).
Gorina, Y. V., Komleva, Yu. K., Lopatina, O. L., et al., “Insulin resistance in the development of impairments of complex forms of behavior and memory in chronic Alzheimer’s-type neurodegeneration,” in: Neurosciences for Medicine and Psychology: 13th Int. Interdisciplinary Congress, Sudak, Crimea, Russia, May 30 – June 10, 2017, pp. 128–129.
Gorina, Y. V., Lopatina, O. L., Komleva, Yu. K., et al., “The role of neuroinflammation in mediating cognitive functions and social interactions in mice with age-dependent neurodegeneration,” Ann. Klin. Eksperim. Nevrol., 12, No. 2, 27–32 (2018).
Gu, L., Wu, D., Tang, X., et al., “Myelin changes at the early stage of 5XFAD mice,” Brain Res. Bull., 137, 285–293 (2018).
Héraud, C., Goufak, D., Ando, K., et al., “Increased misfolding and truncation of tau in APP/PS1/tau transgenic mice compared to mutant tau mice,” Neurobiol. Dis., 62, 100–112 (2014).
Holcomb, L., Gordon, M. N., McGowan, E., et al., “Accelerated Alzheimertype phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes,” Nat. Med., 4, No. 1, 97–100 (1998).
Hüttenrauch, M., Baches, S., Gerth, J., et al., “Neprilysin deficiency alters the neuropathological and behavioral phenotype in the 5XFAD mouse model of Alzheimer’s disease,” J. Alzheimers Dis., 44, No. 4, 1291–1302 (2015).
Jafari, Z., Okuma, M., Karem, H., et al., “Prenatal noise stress aggravates cognitive decline and the onset and progression of beta amyloid pathology in a mouse model of Alzheimer’s disease,” Neurobiol. Aging, 77, 66–86 (2019).
Jaffar, S., Counts, S. E., Ma, et al., “Neuropathology of mice carrying mutant APP (swe) and/or PS1(M146L) transgenes: alterations in the p75(NTR) cholinergic basal forebrain septohippocampal pathway,” Exp. Neurol., 170, No. 2, 227–243 (2001).
Jahn, H., “Memory loss in Alzheimer’s disease,” Dialogues Clin. Neurosci., 15, No. 4, 445–454 (2013).
Janus, C., “Search strategies used by APP transgenic mice during navigation in the Morris water maze,” Learn. Mem., 11, 337–346 (2004).
Johnson, E. C. B., Ho, K., Yu, G. Q., et al., “Behavioral and neural network abnormalities in human APP transgenic mice resemble those of App knock-in mice and are modulated by familial Alzheimer’s disease mutations but not by inhibition of BACE1,” Mol. Neurodegener., 15, No. 1, 53 (2020).
Juszczak, G. R., Sliwa, A. T., Wolak, P., et al., “The usage of video analysis system for detection of immobility in the tail suspension test in mice,” Pharmacol. Biochem. Behav., 85, No. 2, 332–338 (2006).
Kim, H. Y., Lee, D. K., Chung, B.-R., et al., “Intracerebroventricular injection of amyloid-β peptides in normal mice to acutely induce Alzheimer-like cognitive deficits,” J. Vis. Exp., 109, 53308 (2016).
Kitazawa, M., Medeiros, R., and Laferla, F. M., “Transgenic mouse models of Alzheimer disease: developing a better model as a tool for therapeutic interventions,” Curr. Pharm. Des., 18, No. 8, 1131–1147 (2012).
Köhler, C. A., Magalhaes, T. F., Oliveira, J. M. M. P., et al., “Neuropsychiatric disturbances in mild cognitive impairment (MCI, a systematic review of population-based studies,” Curr. Alzheimer Res., 13, No. 10, 1066–1082 (2016).
Koolhaas, J. M., Coppens, C. M., de Boer, S. F., et al., “The resident-intruder paradigm: a standardized test for aggression, violence and social stress,” J. Vis. Exp., 77, e4367 (2013).
Kosel, F., Hamilton, J. S., Harrison, S. L., et al., “Reduced social investigation and increased injurious behavior in transgenic 5xFAD mice,” J. Neurosci. Res. (2020).
Kosel, F., Torres Munoz, P., Yang, J. R., et al., “Age-related changes in social behaviours in the 5xFAD mouse model of Alzheimer’s disease,” Behav. Brain Res., 362, 160–172 (2019).
Lalonde, R., Fukuchi, K., and Strazielle, C., “Neurologic and motor dysfunctions in APP transgenic mice,” Rev. Neurosci., 23, No. 4, 363–379 (2012).
Landel, V., Baranger, K., Virard, I., et al., “Temporal gene profiling of the 5XFAD transgenic mouse model highlights the importance of microglial activation in Alzheimer’s disease,” Mol. Neurodegener., 9, 33 (2014).
Larner, A. J., “Presenilin-1 mutations in Alzheimer’s disease: an update on genotype–phenotype relationships,” J. Alzheimers Dis., 37, No. 4, 653–659 (2013).
Ledo, J. H., Azevedo, E. P., Clarke, J. R., et al., “Correction: Amyloidbeta oligomers link depressive-like behavior and cognitive deficits in mice,” Mol. Psychiatry (2020).
Li, X. Y., Men, W. W., Zhu, H., et al., “Age- and brain region-specific changes of glucose metabolic disorder, learning, and memory dysfunction in early Alzheimer’s disease assessed in APP/PS1 transgenic mice using 18F-FDG-PET,” Int. J. Mol. Sci., 17, No. 10, 1 (2016b).
Li, X., Bao, X., and Wang, R., “Experimental models of Alzheimer’s disease for deciphering the pathogenesis and therapeutic screening,” Int. J. Mol. Med., 37, No. 2, 271–283 (2016a).
Lin, B., Hasegawa, Y., Takane, K., et al., “High-fat-diet intake enhances cerebral amyloid angiopathy and cognitive impairment in a mouse model of Alzheimer’s disease, independently of metabolic disorders,” J. Am. Heart Assoc., 5, No. 6, e003154 (2016).
Lövheim, H., Sandman, P. O., Karlsson, S., and Gustafson, Y., “Behavioral and psychological symptoms of dementia in relation to level of cognitive impairment,” Int. Psychogeriatr., 20, No. 4, 777–789 (2008).
Nyarko, J. N. K., Quartey, M. O., Baker, G. B., and Mousseau, D. D., “Can animal models inform on the relationship between depression and Alzheimer disease?” Can. J. Psychiatry, 64, No. 1, 18–29 (2019).
O’Connor, A., Weston, P. S. J., Pavisic, I. M., et al., “Quantitative detection and staging of presymptomatic cognitive decline in familial Alzheimer’s disease: a retrospective cohort analysis,” Alzheimers Res. Ther., 12, No. 1, 126 (2020).
Oakley, H., Cole, S. L., Logan, S., et al., “Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation,” J. Neurosci., 26, No. 40, 10129–10140 (2006).
Ohno, M., Chang, L., Tseng, W., et al., “Temporal memory deficits in Alzheimer’s mouse models: rescue by genetic deletion of BACE1,” Eur. J. Neurosci., 23, No. 1, 251–260 (2006).
Otvos, L., Jr., Szendrei, G. I., Lee, V. M., and Mantsch, H. H., “Human and rodent Alzheimer beta-amyloid peptides acquire distinct conformations in membrane-mimicking solvents,” Eur. J. Biochem., 211, No. 1–2, 249–257 (1993).
Owona, B. A., Zug, C., Schluesener, H. J., and Zhang, Z.-Y., “Amelioration of behavioral impairments and neuropathology by antiepileptic drug topiramate in a transgenic Alzheimer’s disease model mice, APP/PS1,” Int. J. Mol. Sci., 20, No. 12, 3003 (2019).
Park, S. A., Han, S. M., and Kim, C. E., “New fluid biomarkers tracking non-amyloid-β and non-tau pathology in Alzheimer’s disease,” Exp. Mol. Med., 52, No. 4, 556–568 (2020).
Patel, S., Grizzell, J. A., Holmes, R., et al., “Cotinine halts the advance of Alzheimer’s disease-like pathology and associated depressive-like behavior in Tg6799 mice,” Front. Aging Neurosci., 6, 162 (2014).
Perl, D. P., “Neuropathology of Alzheimer’s disease,” Mt. Sinai J. Med., 77, No. 1, 32–42 (2010).
Petrasek, T., Vojtechova, I., Lobellova, V., et al., “The McGill transgenic rat model of Alzheimer’s disease displays cognitive and motor impairments, changes in anxiety and social behavior, and altered circadian activity,” Front. Aging Neurosci., 10, 250 (2018).
Poe, G. R., Walsh, C. M., and Bjorness, T. E., “Cognitive neuroscience of sleep,” Prog. Brain Res., 185, 1–19 (2010).
Poon, C. H., Wang, Y., Fung, M.-L., et al., “Rodent models of amyloid-beta feature of Alzheimer’s disease: Development and potential treatment implications,” Aging Dis., 11, No. 5, 1235–1259 (2020).
Pratap, A. A. and Holsinge, R. M. D., “Altered brain leptin and leptin receptor expression in the 5XFAD mouse model of Alzheimer’s disease,” Pharmaceuticals (Basel), 13, No. 11, 401 (2020).
Preuss, C., Pandey, R., Piazza, E., et al., “A novel systems biology approach to evaluate mouse models of late-onset Alzheimer’s disease,” Mol. Neurodegener., 15, No. 1, 67 (2020).
Price, J. L., Ko, A. I., Wade, M. J., et al., “Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease,” Arch. Neurol., 58, No. 9, 1395–1402 (2001).
Reinhardt, S., Schuck, F., Grösgen, S., et al., “Unfolded protein response signaling by transcription factor XBP-1 regulates ADAM10 and is affected in Alzheimer’s disease,” FASEB J., 28, No. 2, 978–997 (2014).
Resnick, B., Galik, E., Kolanowski, A., et al., “Gender differences in presentation and management of behavioral and psychological symptoms associated with dementia among nursing home residents with moderate to severe dementia,” J. Women Aging, 1–18 (2020).
Rice, J. P., Wallace, D. G., and Hamilton, D. A., “Lesions of the hippocampus or dorsolateral striatum disrupt distinct aspects of spatial navigation strategies based on proximal and distal information in a cued variant of the Morris water task,” Behav. Brain Res., 289, 105–117 (2015).
Roh, J. H., Jiang, H., Finn, M. B., et al., “Potential role of orexin and sleep modulation in the pathogenesis of Alzheimer’s disease,” J. Exp. Med., 211, No. 3, 2487–2496 (2014).
Sadleir, K. R., Popovic, J., and Vassar, R., “ER stress is not elevated in the 5XFAD mouse model of Alzheimer’s disease,” J. Biol. Chem., 293, No. 48, 18434–18443 (2018).
Samaey, C., Schreurs, A., Stroobants, S., and Balschun, D., “Early cognitive and behavioral deficits in mouse models for tauopathy and Alzheimer’s disease,” Front. Aging Neurosci., 11, 335 (2019).
Scheltens, P., Blennow, K., Breteler, M. M., et al., “Alzheimer’s disease,” Lancet, 388, No. 10043, 505–517 (2016).
Schneider, F., Baldauf, K., Wetzel, W., and Reymann, K. G., “Behavioral and EEG changes in male 5xFAD mice,” Physiol. Behav., 135, 25–33 (2014).
Sethi, M., Joshi, S. S., Webb, R. L., et al., “Increased fragmentation of sleep–wake cycles in the 5XFAD mouse model of Alzheimer’s disease,” Neuroscience, 290, 80–89 (2015).
Sommer, B., Sturchler-Pierrat, C., Abramowski, D., et al., “Transgenic approaches to model Alzheimer’s disease,” Rev. Neurosci., 11, No. 1, 47–51 (2000).
Strassnig, M. and Ganguli, M., “About a peculiar disease of the cerebral cortex: Alzheimer’s original case revisited,” Psychiatry (Edgmont), 2, No. 9, 30–33 (2005).
Volloch, V., Olsen, B., and Rits, S., “Alzheimer’s disease is driven by intraneuronally retained beta-amyloid produced in the AD-specific, βAPP-independent pathway: current perspective and experimental models for tomorrow,” Ann. Integr. Mol. Med., 2, No. 1, 90–114 (2020).
Vorhees, C. V. and Williams, M. T., “Morris water maze: procedures for assessing spatial and related forms of learning and memory,” Nat. Protoc., 1, No. 2, 848–858 (2006).
Walker, J. M., Fowler, S. W., Miller, D. K., et al., “Spatial learning and memory impairment and increased locomotion in a transgenic amyloid precursor protein mouse model of Alzheimer’s disease,” Behav. Brain Res., 222, No. 1, 169–175 (2011).
Wirths, O. and Zampar, S., “Neuron loss in Alzheimer’s disease: Translation in transgenic mouse models,” Int. J. Mol. Sci., 21, No. 21, 8144 (2020).
Xiao, N. A., Zhang, J., Zhou, M., et al., “Reduction of glucose metabolism in olfactory bulb is an earlier Alzheimer’s disease-related biomarker in 5XFAD mice,” Chin. Med. J., 128, No. 16, 2220–2227 (2015).
Xu, W., Xu, F., Anderson, M. E., et al., “Cerebral microvascular rather than parenchymal amyloid-β protein pathology promotes early cognitive impairment in transgenic mice,” J. Alzheimers Dis., 38, No. 3, 621–632 (2014).
Yamazaki, H., Jin, Y., Tsuchiya, A., et al., “Adipose-derived stem cell-conditioned medium ameliorates antidepression-related behaviors in the mouse model of Alzheimer’s disease,” Neurosci. Lett., 609, 53–57 (2015).
Yan, H., Pang, P., Chen, W., et al., “The lesion analysis of cholinergic neurons in 5XFAD mouse model in the three-dimensional level of whole brain,” Mol. Neurobiol., 55, No. 5, 4115–4125 (2018).
Zhang, F., Wei, J., Li, X., et al., “Early candidate urine biomarkers for detecting Alzheimer’s disease before amyloid-β plaque deposition in an APP (swe)/PSEN1dE9 transgenic mouse model,” J. Alzheimers Dis., 66, No. 2, 613–637 (2018).
Zhang, W., Jiao, B., Xiao, T., et al., “Association of rare variants in neurodegenerative genes with familial Alzheimer’s disease,” Ann. Clin. Transl. Neurol., 7, No. 10, 1985–1995 (2020).
Zhao, Q. F., Tan, L., Wang, H. F., et al., “The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: Systematic review and meta-analysis,” J. Affect. Disord., 190, 264–271 (2016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Vysshei Nervnoi Deyatel’nosti imeni I. P. Pavlova, Vol. 71, No. 5, pp. 667–679, September–October, 2021.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gorina, Y.V., Salmina, A.B., Chernyuk, D.P. et al. Features of the Development and Analysis of Impairments to Social Behavior and Cognitive Functions in Animals with Experimental Alzheimer’s Disease. Neurosci Behav Physi 52, 669–676 (2022). https://doi.org/10.1007/s11055-022-01301-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-022-01301-8