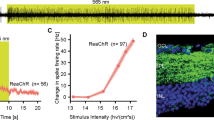
Optogenetics – a method allowing light to be used to control neuron activity via expression of light-activated proteins within cells – is a powerful tool in neurophysiological research. Optogenetics has made significant progress in studies of brain functions in the last decade. Progress in optogenetics depends decisively on the development of new molecular tools, i.e., light-activated proteins. The most widely used molecule for excitation of cells in optogenetics is the natural light-activated cation channelrhodopsin 2 (ChR2). Studies in 2015 identified the natural light-activated chloride channel GtACR2, which in optogenetic experiments can efficiently suppress neuron activity. We identified the unique properties of this channel and showed that not only do GtACR2-expressing neurons respond to the light signal with strong inhibition, but also that action potentials can be generated, evidently in the axon terminals of the neuron due to changes in the chloride reversion potential is this cell compartment. Our studies used optogenetic methods to investigate the cellular mechanisms of learning and memory. Using channelrhodopsin 2 expression in networks of presynaptic neurons, we employed light stimulation to study the properties of synaptic connections and their plasticity in whole populations of neurons in a single experiment. One potential direction in the clinical use of optogenetics is its use for prosthetization of degenerative retinas. One version of this approach consists of creating an ON/OFF receptor field of retinal ganglion cells by targeted expression of the excitatory light-activated protein in the central part of a ganglion cell and an inhibitory protein in the periphery. Within the framework of this approach, we created a two-layer construct carrying genes for excitatory and inhibitory opsins whose expression was able to lead to restoration of ON/OFF interactions typical of ganglion neurons.
Similar content being viewed by others
References
E. S. Boyden, F. Zhang, E. Bamberg, et al., “Millisecond-timescale, genetically targeted optical control of neural activity,” Nat. Neurosci., 8, No. 9, 1263–1268 (2005).
J. Y. Lin, “A user’s guide to channelrhodopsin variants: Features, limitations and future developments,” Exp. Physiol., 96, No. 1, 19–25 (2011).
G. Nagel, T. Szellas, W. Huhn, et al., “Channelrhodopsin-2, a directly light-gated cation-selective membrane channel,” Proc. Natl. Acad. Sci. USA, 100, No. 24, 139,40–13,945 (2003).
J. G. Bernstein and E. S. Boyden, “Optogenetic tools for analyzing the neural circuits of behavior,” Trends Cogn. Sci., 15, No. 12, 592–600 (2011).
A. Berndt, S. Y. Lee, C. Ramakrishnan, and K. Deisseroth, “Structure- guided transformation of channelrhodopsin into a light-activated chloride channel,” Science, 344, 420–424 (2014).
J. Wietek, J. S. Wiegert, N. Adeishvili, et al., “Conversion of channelrhodopsin into a light-gated chloride channel,” Science, 344, 409–412 (2014).
E. G. Govorunova, O. A. Sineshchekov, R. Janz, et al., “Natural light-gated anion channels: A family of microbial rhodopsins for advanced optogenetics,” Science, 349, 647–650 (2015).
D. A. Dolgikh, A. Y. Malyshev, M. V. Roshchin, et al., “Comparative characteristics of two anion-channel rhodopsins and prospects of their use in optogenetics,” Dokl. Biochem. Biophys., 471, 440–442 (2016).
A. Y. Malyshev, M. V. Roshchin, G. R. Smirnova, et al., “Chloride conducting light activated channel GtACR2 can produce both cessation of firing and generation of action potentials in cortical neurons in response to light,” Neurosci. Lett., 640, 76–80 (2017).
M. Mahn, L. Gibor, P. Patil, et al., “High-efficiency optogenetic silencing with soma-targeted anion-conducting channelrhodopsins,” Nat. Commun., 9, 4125 (2018).
M. Chistiakova, N. M. Bannon, M. Bazhenov, and M. Volgushev, “Heterosynaptic plasticity: Multiple mechanisms and multiple roles,” Neuroscientist, 20, 483–98 (2014).
L. M. Langevin, P. Mattar, R. Scardigli, et al., “Validating in utero electroporation for the rapid analysis of gene regulatory elements in the murine telencephalon,” Dev. Dyn., 236, No. 5, 1273–1286 (2007).
N. A. Simonova, N. V. Bal’, P. M. Balaban, et al., “An optogenetic approach to studies of the mechanisms of heterosynaptic plasticity in neocortical neurons,” Zh. Vyssh. Nerv. Deyat., 67, 75–85 (2017).
M. A. Ostrovsky and M. P. Kirpichnikov, “Prospects of optogenetic prosthesis of the degenerative retina of the eye,” Biochemistry (Mosc.), 84, No. 5, 479–490 (2019).
K. P. Greenberg, A. Pham, and F. S. Werblin, “Differential targeting of optical neuromodulators to ganglion cell soma and dendrites allows dynamic control of center-surround antagonism,” Neuron, 69, 713–720 (2011).
C. Wu, E. Ivanova, Y. Zhang, and Z. H. Pan, “rAAV-mediated subcellular targeting of optogenetic tools in retinal ganglion cells in vivo,” PLoS One, 8, e66332 (2013).
E. Y. Koh, S. C. Ho, Mariati, et al., “An internal ribosome entry site (IRES) mutant library for tuning expression level of multiple genes in mammalian cells,” PLoS One, 8, e82100 (2013).
L. E. Petrovskaya, M. V. Roshchin, G. R. Smirnova, et al., “A bicistronic genetic construct for optogenetic prosthetization of the receptive field of ganglion cells in the degenerative retina,” Dokl. Akad. Nauk., 486, 258–261 (2019).
D. A. Dolgikh, A. Y. Malyshev, S. V. Salozhin, et al., “Anionselective channelrhodopsin expressed in neuronal cell culture and in vivo in murine brain: Light-induced inhibition of generation of action potentials,” Dokl. Biochem. Biophys., 465, 424–427 (2015).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 105, No. 11, pp. 1406– 1416, November, 2019.
Rights and permissions
About this article
Cite this article
Malyshev, A.Y., Ostrovsky, M.A. Experience in the Use of Optogenetic Approaches to Studies of Brain Functions and Prosthetization of Degenerative Retinas. Neurosci Behav Physi 50, 1065–1071 (2020). https://doi.org/10.1007/s11055-020-01006-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-020-01006-w