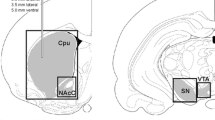
The effects of prenatal stress (PNS) on the formation of the orexinergic and dopaminergic systems of the brain were studied in 14- and 30-day-old rat pups born to females subjected to sleep deprivation (6 h/day) using the “small areas” method from day 13 to day 19 of pregnancy. The open fi eld test showed impairments to motor development in 14-day-old PNS rat pups. Immunohistochemical studies of brain sections showed that the hypothalamus contained a signifi cantly larger quantity of orexin A in neurons in the periforniceal area, while high-performance liquid chromatography demonstrated increases in the dopamine level and its rate of metabolism as compared with control rat pups born to intact females. Western blotting results provided evidence of a decrease in the striatal level of the GABA-sythesizing enzyme (GAD65) and an increase in the level of tyrosine hydroxylase (an enzyme involved in dopamine synthesis) phosphorylated at serine-40 as compared with controls. By day 30 of life measures of motor activity in PNS rat pups were no different from those in controls, though PNS rat pups had higher levels of anxiety and lower levels of exploratory activity. The results of morphological and biochemical studies also provided evidence that there were no differences between the study parameters in the hypothalamus and striatum in 30-day-old rat pups as compared with controls, which was confi rmed by results from electrophysiological studies indicating that there were no differences in the organization of the sleep-waking cycle in 30-day-old PNS and control rat pups. We discuss here the morphofunctional interactions of the orexinergic and dopaminergic systems of the brain in the early postnatal period of development of the body and the role of orexins in the compensatory mechanisms of the brain.
Similar content being viewed by others
References
M. X. Zarrow, J. E. Philpott, and V. H. Denenberg, “Passage of 14C-4-corticosterone from the rat mother to the foetus and neonate,” Nature, 226, No. 5250, 1058–1069 (1970).
M. Weinstock, “Alterations induced by gestational stress in brain morphology and behaviour of the offspring,” Prog. Neurobiol., 65, No. 5, 427–451 (2001).
A. V. V’yushina, A. V. Pritvorova, and M. A. Flerov, “Oxidative modification of proteins in brain structures in Sprague–Dawley rats and some behavioral parameters after prenatal stress,” Neurosci. Behav. Physiol., 44, No. 4, 395–400 (2014).
T. Sakurai, “Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior,” Cell, 92, No. 4, 573–585 (1998).
L. de Lecea, T. S. Kilduff, C. Peyron, et al., “The hypocretins: Hypothalamus- specific peptides with neuroexcitatory activity,” Proc. Natl. Acad. Sci. USA, 95, No. 1, 322–327 (1998).
L. Lin, J. Faraco, R. Li, et al., “The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene,” Cell, 98, No. 3, 365–376 (1999).
R. Chemelli, J. Willie, C. Sinton, et al., “Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation,” Cell, 98, No. 4, 437–451 (1999).
T. Sakurai, T. Moriguchi, K. Furuya, et al., “Structure and function of human prepro-orexin gene,” J. Biol. Chem., 274, No. 25, 17771– 17776 (1999).
T. Sakuraia, “Roles of orexin/hypocretin in regulation of sleep/wakefulness and energy homeostasis,” Sleep Med. Rev., 9, 231–241 (2005).
V. M. Kovalzon, “Central mechanisms of the sleep-wakefulness cycle control,” Human Physiol., 37, No. 4, 500–508 (2011).
V. M. Kovalzon, “Ascending reticular activating system of the brain,” Transl. Neurosci. Clin., 2, No. 4, 275–285 (2016).
E. A. Aristakesian, “Comparative neurophysiological analysis of the waking-sleeping cycle during the early postnatal ontogeny in rats and guinea pigs,” J. Evol. Biochem. Physiol., 33, No. 6, 545–550 (1997).
T. L. Steininger, T. S. Kilduff, M. Behan, et al., “Comparison of hypocretin/orexin and melanin-concentrating hormone neurons and axonal projections in the embryonic and postnatal rat brain,” J. Chem. Neuroanat., 27, No. 3, 165–181 (2004).
Y. Ogawa, T. Kanda, K. Vogt, and M. Yanagisawa, “Anatomical and electrophysiological development of the hypothalamic orexin neurons from embryos to neonates,” J. Comp. Neurol., 525, No. 18, 3809–3820 (2017).
Y. Yamamoto, Y. Ueta, Y. Hara, et al., “Postnatal development of orexin/hypocretin in rats,” Brain Res. Mol. Brain Res., 78, No. 1–2, 108–119 (2000).
A. N. van Den Pol, P. R. Patrylo, P. K. Ghosh, and X. B. Gao, “Lateral hypothalamus: Early developmental expression and response to hypocretin (orexin),” J. Comp. Neurol., 433, No. 3, 349–363 (2001).
L. A. Grafe and S. Bhatnagar, “Orexins and stress,” Front. Neuroendocrinol., 51, 132–145 (2018).
W. J. Giardino and L. de Lecea, “Hypocretin (orexin) neuromodulation of stress and reward pathways,” Curr. Opin. Neurobiol., 29, 103–108 (2014).
N. Ito, T. Yabe, Y. Gamo, et al., “I.c.v. administration of orexin-A induces an antidepressive-like effect through hippocampal cell proliferation,” Neuroscience, 157, No. 4, 720–732 (2008).
K. Bjornstrom, D. Turina, T. Strid, et al., “Orexin A inhibits propofol-induced neurite retraction by a phospholipase D/protein kinase C?-dependent mechanism in neurons,” PLoS One, 9, No. 5, 1–7 (2014).
J. Bakos, M. Zatkova, Z. Bacova, and D. Ostatnikova, “The Role of hypothalamic neuropeptides in neurogenesis and neuritogenesis,” Neural Plast., 2016, 1–10 (2016).
I. Yu. Morina, A. L. Mikhrina, and I. V. Romanova, “Immunohistochemical studies of the pathways of the infl uences of interneuron on orexinergic neurons in really periforniceal area of the rat hypothalamus,” Ros. Fiziol. Zh., 104, No. 6, 692–700 (2018).
T. M. Korotkova, O. A. Sergeeva, K. S. Eriksson, et al., “Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins,” J. Neurosci., 23, No. 1, 7–11 (2003).
E. K. Plowman, N. Maling, B. J. Rivera, et al., “Differential sensitivity of cranial and limb motor function to nigrostriatal dopamine depletion,” Behav. Brain Res., 237, 157–263 (2013).
D. Radl, M. Chiacchiaretta, R. G. Lewis, et al., “Differential regulation of striatal motor behavior and related cellular responses by dopamine D2L and D2S isoforms,” Proc. Natl. Acad. Sci. USA, 115, No. 1, 198–203 (2018).
T. Nakamura, K. Uramura, T. Nambu, et al., “Orexin-induced hyperlocomotion and stereotypy are mediated by the dopaminergic system,” Brain Res., 873, No. 1, 181–187 (2000).
P. G. Svetlov, “The theory of the critical periods of development and its significance for understanding the principles of actions of the environment on ontogeny,” in: Questions in Cytology, USSR Academy of Sciences, Moscow, Leningrad (1966).
V. A. Otellin, L. I. Khozhai, and N. E. Ordyan, Prenatal Stress Actions and the Developing Brain: Adaptive Mechanisms, Immediate and Delayed Effects, Desyatka, St. Petersburg (2007).
R. E. Coggeshall, “A Study of diencephalic development in the albino rat,” J. Comp. Neurol., 122, No. 2, 241–299 (1964).
V. G. Kassil’, V. A. Otellin, L. I. Khozhai, and V. B. Kostkin, “Critical periods in the development of the brain,” Ros. Fiziol. Zh., 86, No. 11, 1418–1425 (2000).
C. Amiot, F. Brischoux, C. Colard, et al., “Hypocretin/orexin-containing neurons are produced in one sharp peak in the developing ventral diencephalon,” Eur. J. Neurosci., 22, No. 2, 531–534 (2005).
A. M. Mandl, “The phases of the estrous cycle in the adult white rat,” J. Exp. Biol., 28, 576–584 (1951).
F. K. Marcondes, F. J. Bianchi, and A. P. Tanno, “Determination of the estrous cycle phases of rats: Some helpful considerations,” Braz. J. Biol., 62, No. 4, 609–614 (2002).
G. W. Vogel, “A review of REM sleep deprivation,” Arch. Gen. Psychiatr., 32, No. 6, 749–761 (1975).
A. M. L. Coenen and E. L. J. M. van Luijtelaar, “Stress induced by three procedures of deprivation of paradoxical sleep,” Physiol. Behav., 35, No. 4, 501–504 (1985).
V. G. Makarov and M. N. Makarova, Handbook. Normal Physiological, Biochemical, and Biometric Parameters of Experimental Animals, LEMA Press, St. Petersburg (2013).
K. L. Lambertsen, J. B. Gramsbergen, M. Sivasaravanaparan, et al., “Genetic KCa3.1-defi ciency produces locomotor hyperactivity and alterations in cerebral monoamine levels,” PLos One, 7, No. 10, 1–15 (2012).
G. Paxinos and C. Watson, The Rat Brain in Stereotaxic Coordinates, Academic Press, San Diego (1998).
I. N. Krasnova, E. R. Bychkov, V. I. Lioudyno, et al., “Intracerebroventricular administration of substance P increases dopamine content in the brain of 6-hydroxydopamine lesioned rats,” Neuroscience, 95, No. 1, 113–117 (2000).
V. M. Koval’zon, and V. L. Tsibul’skii, “Deprivation of REM sleep by stimulation of the reticular formation in rats,” Ros. Fiziol. Zh., 64, No. 8, 1082–1088 (1978).
B. Ya. Ryzhavskii, “Development of the brain in early ontogeny,” Sorosov. Obrazovat. Zh., 6, No. 1, 37–43 (2000).
A. Harris and J. Seckl, “Glucocorticoids, prenatal stress and the programming of disease,” Horm. Behav., 59, No. 3, 279–289 (2011).
E. K. Plowman, N. Maling, B. J. Rivera, et al., “Differential sensitivity of cranial and limb motor function to nigrostriatal dopamine depletion,” Behav. Brain Res., 237, 157–263 (2013).
J. J. Radley, A. B. Rocher, A. Rodriguez, et al., “Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex,” J. Comp. Neurol., 507, No. 1, 1141–1150 (2008).
J. W. Haycock, “Phosphorylation of tyrosine hydroxylase in situ at serine 8, 19, 31 and 40,” J. Biol. Chem., 265, 11,682–11,6911 (1990).
P. R. Dunkley, L. Bobrovskaya, M. E. Graham, et al., “Tyrosine hydroxylase phosphorylation: Regulation and consequences,” J. Neurochem., 91, 1025–1043 (2004).
J. H. Kordower, C. W. Olanow, H. B. Dodiya, et al., “Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease,” Brain, 136, No. 8, 2419–2431 (2013).
S. J. Lussier and H. E. Stevens, “Delays in GABAergic interneuron development and behavioral inhibition after prenatal stress,” Dev. Neurobiol., 76, No. 10, 1078–1091 (2016).
H. E. Stevens, T. Su, Y. Yanagawa, and F. M. Vaccarino, “Prenatal stress delays inhibitory neuron progenitor migration in the developing neocortex,” Psychoneuroendocrinology, 38, 509–521 (2013).
A. J. Rodrigues, P. Leao, J. M. Pego, et al., “Mechanisms of initiation and reversal of drug–seeking behavior induced by prenatal exposure to glucocorticoids,” Mol. Psychiatry, 17, No. 12, 1295–1305 (2012).
D. L. A. van den Hove, H. W. M. Steinbusch, A. Scheepens, et al., “Prenatal stress and neonatal rat brain development,” Neuroscience, 137, No. 1, 145–155 (2006).
H. M. Sickmann, T. S. Arentzen, T. B. Dyrby, et al., “Prenatal stress produces sex-specifi c changes in depression-like behavior in rats: Implications for increased vulnerability in females,” J. Dev. Orig. Health Dis., 6, No. 5, 462–474 (2015).
P. S. Brocardo, F. Boehme, A. Patten, et al., “Anxiety- and depression- like behaviors are accompanied by an increase in oxidative stress in a rat model of fetal alcohol spectrum disorders: Protective effects of voluntary physical exercise,” Neuropharmacology, 62, No. 4, 1607–1618 (2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 105, No. 5, pp. 591–607, May, 2019.
Rights and permissions
About this article
Cite this article
Morina, I.Y., Stankova, E.P. & Romanova, I.V. Effects of Prenatal Stress on the Formation of the Orexinergic System of the Hypothalamus in Rats. Neurosci Behav Physi 50, 607–617 (2020). https://doi.org/10.1007/s11055-020-00942-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-020-00942-x