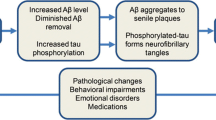
Impairments of sleep and waking in diseases such as Parkinson’s disease, dementia, Lewy body dementia, and Alzheimer’s disease are encountered in a large proportion of patients. Sleep has many functions, supporting the normal operation of the brain, and impairments to sleep may be among the factors promoting the development of neurodegenerative diseases. Impairments to sleep and waking may exacerbate the course of the neurodegenerative process and lead to increases in symptoms, including cognitive impairments. This article presents a detailed analysis of the literature linking sleep and waking impairments and cognitive impairments in neurodegenerative diseases. The phenomenology and methods of correcting sleep and waking impairments in these diseases are discussed.
Similar content being viewed by others
References
L. Xie, H. Kang, Q. Xu, et al., “Sleep drives metabolite clearance from the adult brain,” Science, 342, No. 6156, 373–377 (2013), https://doi.org/10.1126/science.1241224.
S. Bombois, P. Derambure, F. Pasquier, and C. Monaca, “Sleep disorders in aging and dementia,” J. Nutr. Health Aging, 14, No. 3, 212–217 (2010).
D. J. Ley, A. A. Monjan, S. L. Brown, et al., “Sleep complaints among elderly persons: an epidemiologic study of three communities,” Sleep, 18, 425–432 (1995).
Y. E. Ju, J. S. McLeland, C. D. Toedebusch, et al., “Sleep quality and preclinical Alzheimer disease,” JAMA Neurol., 70, No. 5, 587–593 (2013), https://doi.org/10.1001/jamaneurol.2013.2334.
J. Backhaus, J. Born, R. Hoeckesfeld, et al., “Midlife decline in declarative memory consolidation is correlated with a decline in slow wave sleep,” Learn. Mem., 14, No. 5, 336–341 (2007), https://doi.org/10.1101/lm.470507.
J. C. Lo, J. A. Groeger, G. H. Cheng, et al., “Self-reported sleep duration and cognitive performance in older adults: a systematic review and meta-analysis,” Sleep Med., 17, 87–98 (2016), https://doi.org/10.1016/j.sleep.2015.08.021.
J. Benito-Leon, E. D. Louis, and F. Bermejo-Pareja, “Cognitive decline in short and long sleepers: a prospective population-based study (NEDICES),” J. Psychiatr. Res., 47, No. 12, 1998–2003 (2013), https://doi.org/10.1016/j.jpsychires.2013.09.007.
G. Merlino, A. Piani, G. L. Gigli, et al., “Daytime sleepiness is associated with dementia and cognitive decline in older Italian adults: a population-based study,” Sleep Med., 11, No. 4, 372–377 (2010),https://doi.org/10.1016/j.sleep.2009.07.018.
D. Foley, A. Monjan, K. Masaki, et al., “Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men,” J. Am. Ger. Soc., 49, No. 12, 1628–1632 (2001), https://doi.org/10.1111/j.1532-5415.2001.49271.x.
N. E. Cross, J. Lagopoulos, S. L. Duffy, et al., “Sleep quality in healthy older people: relationship with 1H magnetic resonance spectroscopy markers of glial and neuronal integrity,” Behav. Neurosci., 127, No. 5, 803–810 (2013),https://doi.org/10.1037/a0034154.
O. V. Babkina, M. G. Poluektov, and O. S. Levin, “Heterogenity of daytime drowsiness in Parkinson’s disease,” Zh. Nevrol. Psikhiat., 116, No. 6, Iss. 2, 60–70 (2016), https://doi.org/10.17116/jnevro20161166260-70.
G. Tononi and C. Cirelli, “Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration,” Neuron, 81, No. 1, 12–34 (2014).
J. Zhang, Y. Zhu, G. Zhan, et al., “Extended wakefulness: compromised metabolics in and degeneration of locus ceruleus neurons,” J. Neurosci., 34, No. 12, 4418–4431 (2014), https://doi.org/10.1523/JNEUROSCI.5025-12.2014.
M. Matsumae, O. Sato, A. Hirayama, et al., “Research into the physiology of cerebrospinal fl uid reaches a new horizon: intimate exchange between cerebrospinal fl uid and interstitial fl uid may contribute to maintenance of homeostasis in the central nervous system,” Neurol. Med. Chir. (Tokyo), 56, No. 7, 416–441 (2016), https://doi.org/10.2176/nmc.ra.2016-0020.
W. Peng, T. M. Achariyar, B. Li, et al., “Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease,” Neurobiol. Dis., 93, 215–225 (2016).
P. Venkat, M. Chopp, A. Zacharek, et al., “White matter damage and glymphatic dysfunction in a model of vascular dementia in rats with no prior vascular pathologies,” Neurobiol. Aging, 50, 96–106 (2017),https://doi.org/10.1016/j.neurobiolaging.2016.11.002.
D. M. Zeppenfeld, M. Simon, J. D. Haswell, et al., “Association of perivascular loalization of aquaporin-4 with cognition and Alzheimer disease in aging brains,” JAMA Neurol., 74, No. 1, 91 (2017), https://doi.org/10.1001/jamaneurol.2016.4370.
K. G. Burfeind, C. F. Murchison, S. K. Westaway, et al., “The effects of noncoding aquaporin-4 single-nucleotide polymorphisms on cognition and functional progression of Alzheimer’s disease,” Alzheim. Dement. (NY), 3, No. 3, 348–359 (2017), https://doi.org/10.1016/j.trci.2017.05.001.
A. P. Spira, L. P. Chen-Edinboro, M. N. Wu, and K. Yaffe, “Impact of sleep on the risk of cognitive decline and dementia,” Curr. Opin. Psychiatry, 27, No. 6, 478–483 (2014).
H. H. Weng, Y. H. Tsai, C. F. Chen, et al., “Mapping gray matter reductions in obstructive sleep apnea: an activation likelihood estima tion meta-analysis,” Sleep, 37, No. 1, 167–175 (2014), https://doi.org/10.5665/sleep.3330.
A. M. V. Wennberg, M. N. Wu, P. B. Rosenberg, and A. P. Spira, “Sleep disturbance, cognitive decline, and dementia: A review,” Semin. Neurol., 37, No. 4, 395–406 (2017).
K. Gagnon, A. A. Baril, J. F. Gagnon, et al., “Cognitive impairment in obstructive sleep apnea,” Pathol. Biol. (Paris), 62, No. 5, 233–240 (2014), https://doi.org/10.1016/j.patbio.2014.05.015.
C. Lal, C. Strange, and D. Bachman, “Neurocognitive impairment in obstructive sleep apnea,” Chest, 141, No. 6, 1601–1610 (2012), https://doi.org/10.1378/chest.11-2214.
O. S. Levin and N. V. Fedorova, Parkinson’s Disease, MEDpress- Inform (2012), 2nd ed.
A. Ylikoski, K. Martikainen, M. Sieminski, and M. Partinen, “Parkinson’s disease and insomnia,” Neurol. Sci., 36, No. 11, 2003–2010 (2015), https://doi.org/10.1007/s10072-015-2288-9.
E. A. Lyashenko, O. S. Levin, and M. G. Poluektov, “Use of mega for correction of behavioral disorders in the rapid eye movement phase of sleep in Parkinson’s disease,” Zh. Nevrol. Psikhiat., Spec. Issue, 115, No. 6, Iss. 2, 40–43 (2015), https://doi.org/10.17116/jnevro20151156240-43.
Y. E. Kim and B. S. Jeon, “Clinical implication of REM sleep behavior disorder in Parkinson’s disease,” J. Parkinsons Dis., 4, No. 2,237–244 (2014), https://doi.org/10.3233/JPD-130293.
O. V. Babkina, M. G. Poluektov, and O. S. Levin, “ Disruption to circadian regulatory mechanisms in age-dependent neurodegenerative diseases,” Effektiv. Farmakoter., 35, 114–122 (2017).
M. D. Gjerstad, O. B. Tysnes, and J. P. Larsen, “Increased risk of leg motor restlessness but not RLS in early Parkinson disease,” Neurology, 77, No. 22, 1941–1946 (2011), https://doi.org/10.1212/WNL.0b013e31823a0cc8.
S. Kumar, M. Bhatia, and M. Behari, “Sleep disorders in Parkinson’s disease,” Mov. Disord., 4, 775–778 (2002), https://doi.org/10.1002/mds.10167.
X. Y. Zhu, Y. Liu, X. J. Zhang, https://doi.org/10.1212/WNL.0b013e31823a0cc8”Clinical characteristics of leg restlessness in Parkinson’s disease compared with idiopathic Restless Legs Syndrome,” J. Neurol. Sci., 357, No. 1–2, 109–114 (2015), https://doi.org/10.1016/j.jns.2015.07.008.
T. Simuni, C. Caspell-Garcia, C. Coffey, et al., PPMI Sleep Working Group on behalf of the PPMI Investigators, “Correlates of excessive daytime sleepiness in de novo Parkinson’s disease: A case control study,” Mov. Disord., 30, No. 10, 1371–1381 (2015).
S. Kato, H. Watanabe, J. Senda, et al., “Widespread cortical and subcortical brain atrophy in Parkinson’s disease with excessive daytime sleepiness,” J. Neurol., 259, No. 2, 318–326 (2012), https://doi.org/10.1007/s00415-011-6187-6.
O. V. Yakovleva, M. G. Poluektov, O. S. Levin, and E. A. Lyashenko, “Sleep and waking disiorders in neurodegenerative diseases,” Zh. Nevrol. Psikhiat., Spec. Issue, 118, No. 4, 83–91 (2018).
N. J. Diederich, M. Vaillant, M. Leischen, et al., “Sleep apnea syndrome in Parkinson’s disease. A case-control study in 49 patients,” Mov. Disord., 20, No. 11, 1413–1418 (2005).
A. Cagnin, F. Fragiacomo, G. Camporese, et al., “Sleep-wake profi le in dementia with Lewy bodies, Alzheimer’s disease, and normal aging,” J. Alzheim. Dis., 55, No. 4, 1529–1536 (2017), https://doi.org/10.3233/JAD-160385.
I. G. McKeith, B. F. Boeve, D. W. Dickson, et al., “Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium,” Neurology, 89, No. 1, 88–100 (2017).
F. Boddy, E. N. Rowan, D. Lett, et al., “Subjectively reported sleep quality and excessive daytime somnolence in Parkinson’s disease with and without dementia, dementia with Lewy bodies and Alzheimer’s disease,” Int. J. Geriatr. Psychiatry, 22, No. 6, 529–535. (2007).
C. R. Baumann, Y. Dauvilliers, E. Mignot, and C. L. Bassetti, “Normal CSF hypocretin-1 (orexin A) levels in dementia with Lewy bodies associated with excessive daytime sleepiness,” Eur. Neurol.,52, 73–76 (2004).
B. Guarnieri, F. Adorni, M. Musicco, et al., “Prevalence of sleep disturbances in mild cognitive impairment and dementing disorders: a multicenter Italian clinical cross-sectional study on 431 patients,” Dement. Geriatr. Cogn. Disord., 33, No. 1, 50–58 (2012).
S. Hibi, Y. Yamaguchi, Y. Umeda-Kameyama, et al., “The high frequency of periodic limb movements in patients with Lewy body dementia,” J. Psychiatr. Res., 46, 1590–1594 (2012).
J. Yesavage, L. F. Friedman, H. C. Kraemer, et al., “A follow-up study of actigraphic measures in home-residing Alzheimer’s disease patients,” J. Geriatr. Psychiatry Neurol., 11, No. 1, 7–10 (1998),https://doi.org/10.1177/089198879801100103.
C. Schenck, B. Boeve, and M. Mahowald, “Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series,” Sleep Med., 14, No. 8, 744–748 (2013), https://doi.org/10.1016/j.sleep.2012.10.009.
D. P. Cardinali, A. M. Furio, and L. I. Brusco, “Clinical aspects of melatonin intervention in Alzheimer’s disease progression,” Curr. Neuropharmacol., 8, No. 3, 218–227 (2010).
K. E. Moe, M. V. Vitiello, L. H. Larsen, and P. N. Prinz, “Symposium: Cognitive processes and sleep disturbances: Sleep/wake patterns in Alzheimer’s disease: relationships with cognition and function,” J. Sleep Res., 4, No. 1, 15–20 (1995).
L. Volicer, D. G. Harper, B. C. Manning, et al., “Sundowning and circadian rhythms in Alzheimer’s disease,” Am. J. Psychiatry, 158, No. 5, 704–711 (2001), https://doi.org/10.1176/appi.ajp.158.5.704.
T. J. Ferman, G. E. Smith, D. W. Dickson, et al., “Abnormal daytime sleepiness in dementia with Lewy bodies compared to Alzheimer’s disease using the multiple sleep latency test,” Alzheim. Res. Ther., 6, No. 9, 76 (2014), https://doi.org/10.1186/s13195-014-0076-z.
M. Cohen-Zion, C. Stepnowsky, Marler, et al., “Changes in cognitive function associated with sleep disordered breathing in older people,” J. Am. Geriatr. Soc., 49, No. 12, 1622–1627 (2001), https://doi.org/10.1046/j.1532-5415.2001.t01-1-49270.x.
F. Onen and H. Onen, “Obstructive sleep apnea and cognitive impairment in the elderly,” Psychol. Neuropsychiatr. Vieil., 8, 163–169 (2010), https://doi.org/10.1684/pnv.2010.0219.
V. E. Pearson, R. P. Allen, T. Dean, et al., “Cognitive deficits associated with restless legs syndrome (RLS),” Sleep Med., 7, No. 1, 25– 30 (2006), https://doi.org/10.1016/j.sleep.2005.05.006.
S. Hood S and S. Amir, “Neurodegeneration and the circadian clock,” Front. Aging Neurosci., 9, 170 (2017).
G. Dowling, J. Mastick, E. Colling, et al., “Melatonin for sleep disturbances in Parkinson’s disease,” Sleep Med., 6, No. 5, 459–466 (2005), https://doi.org/10.1016/j.sleep.2005.04.004.
C. Medeiros, P. Carvalhedo de Bruin, L. Lopes, et al., “Effect of exogenous melatonin on sleep and motor dysfunction in Parkinson’s disease,” J. Neurol., 254, No. 4, 459–464 (2007), https://doi.org/10.1007/s00415-006-0390-x.
A. de Jonghe, J. Korevaar, B. van Munster, and S. de Rooij, “Effectiveness of melatonin treatment on circadian rhythm disturbances in dementia. Are there implications for delirium? A systematic review,” Int. J. Geriatr. Psychiatry, 25, No. 12, 1201–1208 (2010), https://doi.org/10.1002/gps.2454.
S. Ancoli-Israel, B. W. Palmer, J. R. Cooke, et al., “Cognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: A randomized controlled study,” J. Am. Ger. Soc., 56, No. 11, 2076–2081 (2008), https://doi.org/10.1111/j.1532-5415.2008.01934.x.
D. Kunz and F. Bes, “Melatonin as a therapy in REM sleep behavior disorder patients: an open-labeled pilot study on the possible infl uence of melatonin on REM-sleep regulation,” Mov. Disord., 14, 507–511 (1999).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Nevrologii i Psikhiatrii imeni S. S. Korsakova, Vol. 119, No. 4, Iss. 2, Insomnia, pp. 89–98, April, 2019.
Rights and permissions
About this article
Cite this article
Yakovleva, O.V., Poluektov, M.G., Lyashenko, E.A. et al. Sleep and Cognitive Impairments in Neurodegenerative Diseases. Neurosci Behav Physi 50, 275–282 (2020). https://doi.org/10.1007/s11055-020-00898-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-020-00898-y