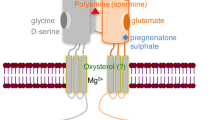
γ-Aminobutyric acid (GABA) is a the main inhibitory transmitter in the central nervous system, which determines the efficiency of neuronal interactions. GABA receptors play a key role in various stages of fear memory – formation, consolidation, retention, reconsolidation, and extinction. Extinction is an important behavioral phenomenon which allows the body to adapt its behavior to changes in the surrounding world. Fear memory extinction constitutes “new” learning, which interferes with the expression of a previously acquired conditioned fear reaction. This review summarizes our own and published data on the GABAergic modulation of fear memory extinction and the potential for pharmacological correction of impairments to this process by actions on GABAA and GABAB receptors.
Similar content being viewed by others
References
Akirav, L., Raizel, H., and Maroun, M., “Enhancement of conditioned fear extinction by infusion of the GABA agonist muscimol into the rat prefrontal cortex and amygdala,” Eur. J. Neurosci., 23, No. 3, 758–764 (2006).
Alberini, C. M., “The role of reconsolidation and the dynamic process of long-term memory formation and storage,” Front. Behav. Neurosci., 5, article 12 (2011).
Amano, T., Unal, C. T., and Paré D., “Synaptic correlates of fear extinction in the amygdala,” Nat. Neurosci., 13, No. 4, 489–494 (2010).
Amikishieva, A. V. and Semedyaeva, S. N., “Effects of baclofen on anxiety, sexual motivation, and olfactory perception in male mice with different psychoemotional status,” Ros. Fiziol. Zh., 92, No. 9, 1122–1134 (2006).
Atack, J. R., “GABAA receptor subtype-selective modulators. I. α2/α3-selective agonists as non-sedating anxiolytics. II. α5-selective inverse agonists for cognition enhancement,” Curr. Top. Behav. Neurosci., 11, No. 9, 1176–1214 (2011).
Auber, A., Tedesco, V., Jones, C. E., et al., “Post-retrieval extinction as reconsolidation interference: methodological issues or boundary conditions?” Psychopharmacology (Berl.)., 226, No. 4, 631–647 (2013).
Baker, K. D., Den, M. L., Graham, B. M., and Richardson R., “A window of vulnerability: Impaired fear extinction in adolescence.” Neurobiol. Learn. Mem., 113, 90–100 (2014).
Berlau, D. J. and McGaugh, J. L., “Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basolateral amygdala,” Neurobiol. Learn. Mem., 86, No. 2, 123–132 (2006).
Bi, L. L., Wang, J., Luo, Z. Y., et al., “Enhanced excitability in the infralimbic cortex produces anxiety-like behaviors,” Neuropharmacology, 72: 148–156 (2013).
Bonardi, C., de Pulford, F., Jennings, D., and Pardon, M. C., “A detailed analysis of the early context extinction deficits seen in APPswe/PS1dE9 female mice and their relevance to preclinical Alzheimer’s disease,” Behav. Brain Res., 222, No. 1, 89–97 (2011).
Bukalo, O., Pinard, C. R., and Holmes A., “Mechanisms to medicines: elucidating neural and molecular substrates of fear extinction to identify novel treatments for anxiety disorders,” Br. J. Pharmacol., 171, No. 20, 4690–4716 (2014).
Bush, D. E., Sotres-Bayon, F., and LeDoux, J. E., “Individual differences in fear: isolating fear reactivity and fear recovery phenotypes,” J. Trauma Stress., 20, No. 4, 413–422 (2007).
Bustos, S. G., Maldonado, H., and Molina, V. A., “Disruptive effect of midazolam on fear memory reconsolidation: decisive influence of reactivation time span and memory age,” Neuropsychopharmacology, 34, No. 2, 446–457 (2009).
Cammarota, M., Revilaqua, L. R. M., Kett, D., et al., “Inhibition of mRNA and protein synthesis in the CA1 region of the dorsal hippocampus blocks reinstallment of an extinguished fear response,” J. Neurosci., 23, No. 3, 737–741 (2003).
Camp, M. C., Macpherson, K. P., Lederle, L., et al., “Genetic strain differences in learned fear inhibition associated with variation in neuroendocrine, autonomic, and amygdala dendritic phenotypes,” Neuropsychopharmacology, 37, No. 6, 1534–1547 (2012).
Chauveau, F., Lange, M. D., Jüngling K., et al., “Prevention of stress-impaired fear extinction through neuropeptide s action in the lateral amygdala,” Neuropsychopharmacology, 37, No. 7, 1588–1599 (2012).
Chhatwal, J. P., Myers, K. M., Ressler, K. J., and Davis, M., “Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction,” J. Neurosci., 25, No. 2, 502–506 (2005).
Choi D. C., Rothbaum, B. O., Gerardi, M., and Ressler, K. J., “Pharmacological enhancement of behavioral therapy: focus on posttraumatic stress disorder,” Curr. Top. Behav. Neurosci., 2, 279–299 (2010).
Corcoran, K. A., Desmond, T. J., Frey, K. A., and Maren S., “Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction,” J. Neurosci., 25, No. 39, 8978–8987 (2005).
Cryan, J. F. and Slattery, D. A., “GABAB receptors and depression. Current status,” Adv. Pharmacol., 58, 427–451 (2010).
Davis, M., “NMDA receptors and fear extinction: implications for cognitive behavioral therapy,” Dialogues Clin. Neurosci., 13, No. 4, 463–474 (2011).
Davis, M., Walker, D. L., Miles, L., and Grillon, C., “Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety,” Neuropsychopharmacology, 35, No. 1, 105–135 (2010).
Delamater, A. R. and Westbrook, R. F., “Psychological and neural mechanisms of experimental extinction: A selective review,” Neurobiol. Learn. Mem., 108C: 38–51 (2014).
DiSorbo, A., Wilson, G. N., Bacik, S., et al., “Time-dependent retrograde amnesic effects of muscimol on conditioned taste aversion extinction,” Pharmacol. Biochem. Behav., 92, No. 2, 319–326 (2009).
Dobi, A., Sartori, S. B., Busti, D., et al., “Neural substrates for the distinct effects of presynaptic group III metabotropic glutamate receptors on extinction of con textual fear conditioning in mice,” Neuropharmacology, 66, 274–289 (2013).
DuBois, D. W., Perlegas, A., Floyd, D. W., et al., “Distinct functional characteristics of the lateral/basolateral amygdala GABAergic system in C57BL/6J and DBA/2J mice,” J. Pharmacol. Exp. Ther., 318, No. 2, 629–640 (2006).
Dubrovina, N. I. and Red’kina, A. V., “Latent inhibition and extinction of a conditioned passive avoidance reaction in C57BL/6J and DBA/2J mice,” Ros. Fiziol. Zh., 98, No. 4, 488–496 (2012).
Dubrovina, N. I. and Zinov’ev, D. R., “The contribution of GABA receptors to extinction of memory traces in health and depression-like states,” Ros. Fiziol. Zh., 93, No. 11, 1285–1291 (2007).
Dubrovina, N. I., “Extinction of fear memories in experimental models of depression,” Usp. Fiziol. Nauk., 42, No. 1, 53–66 (2011).
Dubrovina, N. I., Zinov’ev, D. R., and Zinov’ev, D. V., “Extinction of a conditioned passive avoidance reaction in different mouse strains,” Zh. Vyssh. Nerv. Deyat., 60, No. 6, 712–718 (2010).
Dubrovina, N. I., Zinov’ev, D. R., Zinov’eva, D. V., and Kulikov, A. V., “Learning and extinction of a conditioned passive avoidance reaction in mice with high predisposition to catalepsy,” Ros. Fiziol. Zh., 94, No. 6, 608–616 (2008).
Dunlap B. W., Mansson, E., and Gerardi, M., “Pharmacological innovations for posttraumatic stress disorder and medication- enhanced psychotherapy,” Curr. Pharm. Des., 18, No. 35, 5645–5658 (2012).
Ehrlich, I., Humeau, Y., Grenier, F., et al., “Amygdala inhibitory circuits and the control of fear memory,” Neuron., 62, No. 6, 757–771 (2009).
Engin, E., Lin, J., and Rudolph U., “α2-containing GABA(A) receptors: a target for the development of novel treatment strategies for CNS disorders,” Pharmacol. Ther., 136, No. 2, 142–152 (2012).
Fitzgerald, P. J., Seemann J., R., and Maren S., “Can fear extinction be enhanced? A review of pharmacological and behavioral findings,” Brain Res. Bull., 105, 46–60 (2014).
Frankowska, M., Filip, M., and Przegaliński, E., “Effects of GABAB receptor ligands in animal tests of depression and anxiety,” Pharmacol. Rep., 59, No. 6, 645–655 (2007).
Gafford, G. M., Guo, J. D., Flandreau, E. I., et al., “Cell-type specific deletion of GABA(A)α1 in corticotropin-releasing factor-containing neurons enhances anxiety and disrupts fear extinction,” Proc. Natl. Acad. Sci. USA., 109, No. 40, 16330–16335 (2012).
Ganella, D. E. and Kim, J. H., “Developmental rodent models of fear and anxiety: From neurobiology to pharmacology,” Br. J. Pharmacol., 171, No. 20, 4556–4574 (2014).
Ghose, S., Winter, M. K., McCarson, K. E., et al., “The GABAB receptor as a target for antidepressant drug action,” Br. J. Pharmacol., 162, No. 1, 1–17 (2011).
Gonnan, J. M. and Roose, S. P., “The neurobiology of fear memory reconsolidation and psychoanalytic theory,” J. Am. Psychoanal. Assoc., 59, No. 6, 1201–1220 (2011).
Graham, B. M., Langton, J. M., and Richardson, R., “Pharmacological enhancement of fear reduction: preclinical models,” Br. J. Pharmacol., 164, No. 4, 1230–1247 (2011).
Grigor’yan, G. A. and Markevich, V. A., “Consolidation, reactivation, and reconsolidation of memories,” Zh. Vyssh. Nerv. Deyat., 64, No. 2, 123–136 (2014).
Hart, G., Panayi, M. C., Harris, J. A., and Westbrook, R. F., “Benzodiazepine treatment can impair or spare extinction, depending on when it is given,” Behav. Res. Ther., 56, 22–29 (2014).
Hart, O., Harris, J. A., and Westbrook, R. F., “Systemic or intraamygdala infusion of the benzodiazepine, midazolam, impairs learning, but facilitates re-learning to inhibit fear responses in extinction,” Learn. Mem., 17, No. 4, 210–220 (2010).
Hartley, C. A. and Phelps, E. A., “Changing fear: the neurocircuitry of emotion regulation,” Neuropsychopharmacology, 35, No. 1, 136–146 (2010).
Heaney, C. E., Bolton, M. M., Murtishaw, A. S., et al., “Baclofen administration alters fear extinction and GABAergic protein levels,” Neurobiol. Learn. Mem., 98, No. 3, 261–271 (2012).
Hefner, K., Whittle, N., Juhasz, J., et al., “Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain,” J. Neurosci., 28, No. 32, 8074–8085 (2008).
Heinrichs, S. C., Leite-Morris, K. A., Carey, R. J., and Kaplan, G. B., “Baclofen enhances extinction of opiate conditioned place preference,” Behav. Brain Res., 207, No. 2, 353–359 (2010).
Heldt, S. A. and Ressler, K. J., “Training-induced changes in the expression of GABAA-associated genes in the amygdala after the acquisition and extinction of Pavlovian fear,” Eur. J. Neurosci., 26, No. 12, 3631–3644 (2007).
Heldt, S. A., Mou, L., and Ressler, K. J., “In vivo knockdown of GAD67 in the amygdala disrupts fear extinction and the anxiolytic-like effect of diazepam in mice,” Transl. Psychiatry, 2, e181 (2012).
Herry, C., Ferraguti, F., Singewald, N., et al., “Neuronal circuits of fear extinction,” Eur. J. Neurosci., 31, No. 4, 599–612 (2010).
Hobin, J. A., Ji, J., and Maren, S., “Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats,” Hippocampus, 16, No. 2, 174–182 (2006).
Hofmann, S. G., Fang, A., and Gainer, C. A., “Cognitive enhancers for the treatment of anxiety disorders,” Restor. Neurol. Neurosci., 32, No. 1, 183–195 (2014).
Holt, D. J., Lebron-Milad K, Milad, M. R., et al., “Extinction memory is impaired in schizophrenia,” Biol. Psychiatry, 65, No. 6, 455–463 (2009).
Izquierdo, A., Wellman, C. L., and Holmes, A., “Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice,” J. Neurosci., 26, No. 21, 5733–5738 (2006).
Jacobson, L. H., Kelly P. H., Bettler, B., et al., “GABAB(1) receptor isoforms differentially mediate the acquisition and extinction of aversive taste memories,” J. Neurosci., 26, No. 34, 8800–8803 (2006).
Jiao, X., Pang, K. C., Beck, K. D., et al., “Avoidance perseveration during extinction training in Wistar-Kyoto rats: An interaction of innate vulnerability and stressor intensity,” Behav. Brain Res., 221, No. 1, 98–107 (2011).
Johnston, G. A., Chebib, M., Hanrahan, J. R., and Mewett, K. N., “Neurochemicals for the investigation of GABA(C) receptors,” Neurochem. Res., 35, No. 12, 1970–1977 (2010).
Kaluev, A. V. and Nutt, D. J., “The role of GABA in the pathogenesis of anxiety and depression,” Eskperim. Klin. Farmakol., 67, No. 4, 71–76 (2004).
Kaplan, G. B. and Moore, K. A., “The use of cognitive enhancers in animal models of fear extinction,” Pharmacol. Biochem. Behav., 99, No. 2, 217–228 (2011).
Kim, J. H. and Richardson, R., “New findings on extinction of conditioned fear early in development: theoretical and clinical implications,” Biol. Psychiatry, 67, No. 4, 297–303 (2010).
King E. C., Pattwell, S. S., Glatt C. E., and Lee, F. S., “Sensitive periods in fear learning and memory,” Stress, 17, No. 1, 13–21 (2014).
Kumar, K., Sharma, S., Kumar, R., and Deshmukh, R., “Therapeutic potential of GABA(B) receptor ligands in drug addiction, anxiety, depression and other CNS disorders,” Pharmacol. Biochem. Behav., 110, 174–184 (2013).
Lattal, K. M. and Maughan, D. K., “A parametric analysis of factors affecting acquisition and extinction of contextual fear in C57BL/6 and DBA/2 mice,” Behav. Processes, 90, No. 1, 49–57 (2012).
Lehner, M., Wisłowska-Stanek, A., Taracha, E., et al., “The effects of midazolam and D-cycloserine on the release of glutamate and GABA in the basolateral amygdala of low and high anxiety rats during extinction trial of a conditioned fear test,” Neurobiol. Learn. Mem., 94, No. 4, 468–480 (2010).
Lei, Y., Yaroslavsky, I., and Tejani-Butt, S. M., “Strain differences in the distribution of N-methyl-d-aspartate and gamma (gamma)-aminobutyric acid-A receptors in rat brain,” Life Sci, 85, No. 23–26, 794–799 (2009).
Li, X., Risbrough, V. B., Cates-Gatto, C., et al., “Comparison of the effects of the GABAB receptor positive modulator BHF 177 and the GABAB receptor agonist baclofen on anxiety-like behavior, learning, and memory in mice,” Neuropharmacology, 70, 156–167 (2013).
Lin, H. C., Mao, S. C., and Gean, P. W., “Block of gamma-aminobutyric acid-A receptor insertion in the amygdala impairs extinction of conditioned fear,” Biol. Psychiatry, 66, No. 7, 665–673 (2009).
Liu, G. X., Cai, G. Q., Cai, K. Q., et al., “Reduced anxiety and depression-like behaviors in mice lacking GABA transporter subtype 1,” Neuropsychopharmacology, 32, 1531–1539 (2007).
Lopes, A. P., Ganzer, L., Borges, A. C., et al., “Effects of GABA ligands injected into the nucleus accumbens shell on fear/anxiety-like and feeding behaviours in food-deprived rats,” Pharmacol. Biochem. Behav., 101, No. 1, 41–48 (2012).
Luscher, B., Shen, Q., and Sahir, N., “The GABAergic deficit hypothesis of major depressive disorder,” Mol. Psychiatry, 16, No. 4, 383–406 (2011).
Macpherson, K., Whittle, N., Camp, M., et al., “Temporal factors in the extinction of fear in inbred mouse strains differing in extinction efficacy,” Biol. Mood Anxiety Disord., 3, No. 1, 13 (2013).
Mahler, H., “The GABA system in anxiety and depression and its therapeutic potential,” Neuropharmacology, 62, No. 1, 42–53 (2012).
Makkar, S. R., Zhang, S. Q., and Cranney, J., “Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory,” Neuropsychopharmacology, 35, No. 8, 1625–1652 (2010).
Mamiya, N., Fukushima, H., Suzuki, A., et al., “Brain region-specific gene expression activation required for reconsolidation and extinction of contextual fear memory,” J. Neurosci., 29, No. 2, 402–413 (2009).
Marek, R., Strobel, C., Bredy T. W., and Sah P., “The amygdala and medial prefrontal cortex: partners in the fear circuit,” J. Physiol., 591, Pt. 10, 2381–2391 (2013).
Marin, M. F., Camprodon, I. A., Daugherty, D. D., and Milad, M. R., “Dev icebased brain stimulation to augment fear extinction: implications for PTSD treatment and beyond,” Depress. Anxiety, 31, No. 4, 269–278 (2014).
Marsicano, G., Wotjak, C., T., Azad, S. C., et al., “The endogenous cannabinoid system controls extinction of aversive memories,” Nature, 418, No. 6897, 530–534 (2002).
Mathiasen, L. S., Mirza, N. R., and Rodgers, R. J., “Strain- and model-dependent effects of chlordiazepoxide, L838,417 and zolpidem on anxiety-like behaviours in laboratory mice,” Pharmacol. Biochem. Behav., 90, No. 1, 19–36 (2008).
McEown, K. and Treit, D., “α2 GABAA receptor sub-units in the ventral hippocampus and α5 GABAA receptor sub-units in the dorsal hippocampus mediate anxiety and fear memory,” Neuroscience, 252, 169–177 (2013).
Meng, S., Quan, W., Qi, X., et al., “Effect of baclofen on morphine-induced conditioned place preference, extinction, and stress-induced reinstatement in chronically stressed mice,” Psychopharmacology (Berl.)., 231, No. 1, 27–36 (2014).
Milad, M. R. and Quirk, G. J., “Fear extinction as a model for translational neuroscience: ten years of progress,” Annu. Rev. Psychol., 63, 129–151 (2012).
Miracle, A. D., Brace, M. E., Huyek, K. D., et al., “Chronic stress impairs recall of extinction of conditioned fear,” Neurobiol. Learn. Mem., 85, No. 3, 213–218 (2006).
Mombereau, C., Kaupmann, K., Froestl, W., et al., “Genetic and pharmacological evidence of a role GABA(B) receptors in the modulation of anxiety-and antidepressant-like behavior,” Neuropsychopharmacology, 29, No. 6, 1050–1062 (2004).
Morawska, M. M. and Fendt, M., “The effects of muscimol and AMN082 injections into the medial prefrontal cortex on the expression and extinction of conditioned fear in mice,” J. Exp. Biol., 215, Pt. 8, 1394–1398 (2012).
Morrison, F. G. and Ressler, K. J., “From the neurobiology of extinction to improved clinical treatments.” Depress. Anxiety, 31, No. 4, 279–290 (2014).
Muneoka, K., Shirayama, Y., Horio, M., et al., “Differential levels of brain amino acids in rat models presenting learned helplessness or nonlearned helplessness. Psychopharmacology (Berl.), 229, No. 1, 63–71 (2013).
O’Mahony, C. M., Clarke, O., Gibney, S., et al., “Strain differences in the neurochemical response to chronic restraint stress in the rat: relevance to depression,” Pharmacol. Biochem. Behav., 97, No. 4, 690–699 (2011).
Pare, D. and Duvarci, S., “Amygdala microcircuits mediating fear expression and extinction,” Curr. Opin. Neurobiol., 22, No. 4, 717–723 (2012).
Pattwell, S. S., Lee, E. S., and Casey, B. J., “Fear learning and memory across adolescent development,” Horm. Behav., Spec. Iss., Puberty and Adolescence, 64, No. 2, 380–389 (2013).
Pavlova, I. V. and Rysakova, M. P., “Effects of administration of an agonist and an antagonist of GABAA receptors into the basolateral nucleus of the amygdala on the manifestation and extinction of fear in rats with different durations of freezing,” Zh. Vyssh. Nerv. Deyat., 64, No. 4, 460–473 (2014).
Paydar, A., Lee, B., Gangadharan, G., et al., “Extrasynaptic GABAA receptors in mediodorsal thalamic nucleus modulate fear extinction learning,” Mol. Brain, 7, No. 1, 39 (2014).
Perfilova, V. N. and Tyurenkov, I. N., “GABAC receptors: structure and function,” Eskperim. Klin. Farmakol., 74, No. 1, 45–49 (2011).
Perrine, S. A., Ghoddoussi, F., Michaels, M. S., et al., “Ketamine reverses stress-induced depression-like behavior and increased GABA levels in the anterior cingulate: An 11.7T 1H-MRS study in rats,” Prog. Neuropsychopharmacol. Biol. Psychiatry, 51, 9–15 (2014).
Pilc, A. and Nowak G., “GABAergic hypotheses of anxiety and depression: focus on GABA-B receptors,” Drug Today, 41, No. 11, 755–766 (2005).
Pinard, A., Seddik, R., and Bettler, B., “GABAB receptors: physiological functions and mechanisms of diversity,” Adv. Pharmacol., 58, 231–255 (2010).
Pinna, G. and Rasmusson, A. M., “Ganaxolone improves behavioral deficits in a mouse model of post-traumatic stress disorder,” Front. Cell Neurosci., 8, 256 (2014).
Psotta, L., Lessmann, V., and Endres, T., “Impaired fear extinction learning in adult heterozygous BDNF knockout mice,” Neurobiol. Learn. Mem., 103, 34–38 (2013).
Ren, Z., Sahir, N., Murakami, S., et al., “Defects in dendrite and spine maturation and synaptogenesis associated with an anxious-depressive-like phenotype of GABAA receptor-deficient mice,” Neuropharmacology, 88, 171–179 (2015).
Sangha, S., Ilenseer, J., Sosulina, L., et al., “Differential regulation of glutamic acid decarboxylase gene expression after extinction of a recent memory vs. intermediate memory,” Learn. Mem., 19, No. 5, 194–200 (2012).
Sangha, S., Narayanan, R. T., Bergado-Acosta, J. R., et al., “Deficiency of the 65 kDa isoform of glutamic acid decarboxylase impairs extinction of cued but not contextual fear memory,” J. Neurosci., 29, No. 50, 15713–15720 (2009).
Sardari, M., Rezayof, A., Khodagholi, F., and Zarrindast, M. R., “Basolateral amygdala GABA-A receptors mediate stress-induced memory retrieval impairment in rats,” Int. J. Neuropsychopharmacol., 17, No. 4, 603–612 (2014).
Schechter, L. E., Ring, R. H., Beyer, C. E., et al., “Innovative approaches for the development of antidepressant drugs: current and future strategies,” NeuroRX, 2, No. 4, 590–611 (2005).
Schimanski, L. A., Wahlsten, D., and Nguyen, P. V., “Selective modification of short-term hippocampal synaptic plasticity and impaired memory extinction in mice with a congenitally reduced hippocampal commissure,” J. Neurosci., 22, No. 18, 8277–8286 (2002).
Shul’gin, G. I., “Neurophysiological and neurotransmitter support of the inhibition of behavior in health and pathological conditions,” Zh. Vyssh. Nerv. Deyat., 60, No. 6, 643–656 (2010).
Sierra-Mercado, D., Padilla-Coreano, N., and Quirk, G. J., “Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear,” Neuropsychopharmacology, 36, No. 2, 529–538 (2011).
Singewald, N., Schmuckermair, C., Whittle, N., et al., “Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders,” Pharmacol. Ther., 149, 150–190 (2015).
Smith, K. S. and Rudolph U., “Anxiety and depression: Mouse genetics and pharmacological approaches to the role of GABA(A) receptor subtypes,” Neuropharmacology, 62, No. 1, 54–62 (2012).
Smith, K. S., Engin, E., Meloni, E. G., and Rudolph, U., “Benzodiazepineinduced anxiolysis and reduction of conditioned fear are mediated by distinct GABAA receptor subtypes in mice,” Neuropharmacology, 63, No. 2, 250–258 (2012).
Sweeney, F. F., O’Leary, O. F., and Cryan, J. F., “Activation but not blockade of GABAB receptors during early life alters anxiety in adulthood in BALB/c mice,” Neuropharmacology, 81, 303–310 (2014).
Sweeney, F. F., O’Leary, O. F., and Cryan, J. F., “GABAB receptor ligands do not modify conditioned fear responses in BALB/c mice,” Behav. Brain Res., 256, 151–156 (2013).
Tasan, R. O., Bukovac, A., Peterschmitt, Y. N., et al., “Altered GABA transmission in a mouse model of increased trait anxiety,” Neuroscience, 183, 71–80 (2011).
Thompson, B. M., Baratta M. V., Biedenkapp, J. C., et al., “Activation of the infralimbic cortex in a fear context enhances extinction learning,” Learn Mem., 17, No. 11, 591–599 (2010).
Todd, T. P., Vurbic, D., and Bouton, M. E., “Behavioral and neurobiological mechanisms of extinction in Pavlovian and instrumental learning,” Neurobiol. Learn. Mem., 108C, 52–64 (2014).
Tronson, N. C., Schrick, C., Fischer, A., et al., “Regulatory mechanisms of fear extinction and depression-like behavior,” Neuropsychopharmacology, 33, No. 7, 1570–1583 (2008).
Van Elzakker, M. B., Dahlgren M. K., Davis, F. C., et al., “From Pavlov to PTSD: The extinction of conditioned fear in rodents, humans, and anxiety disorders,” Neurobiol. Learn. Mem., 113, 3–18 (2013).
Veeraiah, P., Noronha, J. M., Maitra, S., et al., “Dysfunctional glutamatergic and y-aminobutyric acidergic activities in prefrontal cortex of mice in social defeat model of depression,” Biol. Psychiatry, 76, No. 3, 231–238 (2014).
Venault, P., Beracochea, D., Valleau, M., et al., “Mouse lines selected for difference in sensitivity to beta-CCM also differ in memory processes,” Behav. Brain Res., 173, No. 2, 282–287 (2006).
Venzala, E., Garcia-García, A. L., Elizalde, N., and Tordera, R. M., “Social vs. environmental stress models of depression from a behavioural and neurochemical approach,” Eur. Neuropsychopharmacol., 23, No. 7, 697–708 (2013).
Verleye, M., Dumas, S., Heulard, I., et al., “Differential effects of etifoxine on anxiety-like behaviour and convulsions in BALB/cByJ and C57BL/6J mice: any relation to overexpression of central GABAA receptor beta2 subunits?” Eur. Neuropsychopharmacol., 21, No. 6, 457–470 (2011).
Voigt, R. M., Herrold, A. A., and Napier, T. C., “Baclofen facilitates the extinction of methamphetamine-induced conditioned place preference in rats,” Behav. Neurosci., 125, No. 2, 261–267 (2011).
Vollenweider, I., Smith, K. S., Keist, R., and Rudolph, U., “Antidepressantlike properties of α2-containing GABA(A) receptors,” Behav. Brain Res., 217, No. 1, 77–80 (2011).
Whittle, N., Schmuckermair, C., Gunduz Cinar, O., et al., “Deep brain stimulation, histone deacetylase inhibitors and glutamatergic drugs rescue resistance to fear extinction in a genetic mouse model,” Neuropharmacology, 64, 414–423 (2013).
Willner, P., Scheel-Krüger, J., and Belzung, C., “The neurobiology of depression and antidepressant action,” Neurosci. Biobehav. Rev., 37, No. 10, Pt. 1, 2331–2371 (2013).
Wilson, C. A., Vazdarjanova, A., and Terry A. V., Jr., “Exposure to variable prenatal stress in rats: effects on anxiety-related behaviors, innate and contextual fear, and fear extinction,” Behav. Brain Res., 238, 279–288 (2013).
Wiltgen, B. J., Godsil, B. P., Peng, Z., et al., “The alpha1 subunit of the GABA(A) receptor modulates fear learning and plasticity in the lateral amygdala,” Front. Behav. Neurosci., 3, 37 (2009).
Yee, B. K., Hauser, J., Dolgov, V. V., et al., “GABA receptors containing the alpha5 subunit mediate the trace effect in aversive and appetitive conditioning and extinction of conditioned fear,” Eur. J. Neurosci., 20, No. 7, 1928–1936 (2004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Vysshei Nervnoi Deyatel’nosti imeni I. P. Pavlova, Vol. 66, No. 2, pp. 131–147, March–April, 2016.
Rights and permissions
About this article
Cite this article
Dubrovina, N.I. GABA Receptors in the Modulation of Fear Memory Extinction. Neurosci Behav Physi 47, 573–584 (2017). https://doi.org/10.1007/s11055-017-0438-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-017-0438-7