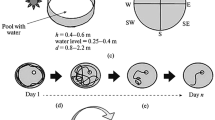
We present here a review of the use of the Morris water maze to identify impairments to the cognitive functions of the brain as part of the evaluation of the toxic actions of nanoparticles. Model experiments showed that individual variability in animals’ behavior has significant influences on the results obtained in the water test. The need for preliminary selection of individuals as a measure to reduce such influences is grounded and the type of behavior displayed by the animal in the test to be used as the criterion for selection is discussed.
Similar content being viewed by others
References
L. F. Abaeva, V. I. Shumskii, E. N. Petritskaya, et al., “Nanoparticles and nanotechnology today and tomorrow,” Alm. Klin. Med., 22, 10–17 (2010).
A. N. Antsiferova, Yu. P. Buzulukov, V. A. Demin, et al., “Radioactive indictor methods and neutron activation nay or studies of the biokinetics of nanoparticles in the living body,” Ros. Nanotekhnol., 10, No. 1–2, 100–108 (2015).
I. V. Gmoshinskii, S. A. Khotimchenko, V. O. Popov, et al., “Nanoma terials and nanotechnology: analysis and monitoring methods,” Usp. Khim., 82, No. 1, 48–76 (2013).
D. Goleman, Emotional Intelligence [Russian translation], AST, Moscow (2010).
D. A. Zhukov, Stop, Who’s Leading? The Biology of Humans and Other Animals, Alpina Non-Fiction, Moscow (2014).
N. F. Izmerov, A. V. Tkach, and L. A. Ivanova, “Nanotechnology and nanoparticles – the state of the problem and tasks for occupational medicine,” Med. Truda Promyshl. Ekol., No. 8, 1–5 (2007).
I. Ya. Podol’skii and I. V. Shchegov, “Effects of suppressing protein ynthesis in the central nervous system on the formation of longterm memory in solving various behavioral tasks,” Zh. Vyssh. Nerv. Deyat., 54, No. 1, 59–67 (2004).
O. A. Solov’eva, Z. I. Storozheva, A. T. Proshin, and V. V. Sherstnev, “Effects of the neurogenesis stimulator Ro 25-6981 on the formation of a spatial skill in adult rats depends on the time of administration and the animals’ learning ability,” Ros. Fiziol. Zh., 97, No. 2, 146–154 (2011).
R. Tomilenko and N. Dubrovina, “Selectivity of the effects of dizocilpine on spatial learning in low- and high-anxiety mice,” Byull. Sib. Otdel. Ros. Akad. Med. Nauk, No. 1, 97–102 (2007).
D. Bucci, A. Chiba, and M. Gallagher, “Spatial learning in male and female Long–Evans rats,” Behav. Neurosci., 109, No. 1, 180–183 (1995).
O. Buresova, E. Panakhova, and J. Bures, “Post-trial fl icker stimulation interferes with spatial memory in the Morris water maze,” Neurosci. Lett., 56, 359–363 (1985).
C. Consalvi, “Motivation and learning in a water maze,” Psychon. Sci., 16, No. 1, 34–35 (1969).
J. Crawley, “Exploratory behavior models of anxiety in mice,” Neurosci. Biobehav. Rev., 9, 37–44 (1985).
D. Van Dam, G. Lenders, and P. De Deyn, “Effect of Morris water maze diameter on visual-spatial learning in different mouse strains,” Neurobiol. Learn. Mem., 85, 164–172 (2006).
J. Daniel, S. Roberts, and G. Dohanich, “Effects of ovarian hormones and environment on radial maze and water maze performance of female rats,” Physiol. Behav., 66, 11–20 (1999).
R. D’Hooge and P. De Deyn, “Applications of the Morris water maze in the study of learning and memory,” Brain Res. Rev., 36, 60–90 (2001).
G. Diana, M. Domenico, A. Loizzo, et al., “Age and strain differences in rat place learning and hippocampal dentate gyrus frequency-potentiation,” Neurosci. Lett., 171, 113–116 (1994).
H. Eichenbaum, C. Stewart, and R. Morris, “Hippocampal representation in place learning,” J. Neurosci., 10, 3531–3542 (1990).
S. Evans, “How rats learn the simple alternation problem in a temporal water maze,” Pedag. Seminary and J. Genetic Psychol., 50, No. 2, 243–275 (1937).
M. Gallagher and M. Nicolle, “Animal models of normal aging, relationship between cognitive decline and markers in hippocampal circuitry,” Behav. Brain Res., 57, 155–162 (1993).
A. Garthe, J. Behr, and G. Kempermann, “Adult-generated hippocampal neurons allow the fl exible use of spatially precise learning strategies,” PLoS One, 4:e546. doi:10.1371/journal.pone.0005464 (2009).
J.-F. Ge, C.-C. Qi, J.P. Qiao, et al., “Sex differences in ICR mice in the Morris water maze task,” Physiol. Res., 62, 107–117 (2013).
Y. Geinisman, L. Detoleddo-Morrell, F. Morrell, and R. Heller, “Hippocampal markers of aged-related memory dysfunction: behavioral, electrophysiological and morphological perspectives,” Prog. Neurobiol., 45, 223–252 (1995).
O. Glaser, “The formation of habits at high speed,” J. Comp. Neurol., 20, 165–184 (1910).
N. Van Goethem, K. Rutten, F. J. can der Staay, et al., “Object recognition testing: Rodent species, strains, housing conditions, and estrous cycle,” Behav. Brain Res., 232, No. 2, 323–334 (2012).
H. Gordon and P. Lee, “No difference in cognitive performance between phases of the menstrual cycle,” Psychoneuroendocrinology, 18, No. 7, 521–531 (1993).
A. Gouveia, U. dos Santos, F. Felisbino, et al., “Infl uence of the estrous cycle on the behavior of rats in the elevated T-maze,” Behav. Proc., 67, No. 2, 167–171 (2004).
A. Gouveia, T. Afonseca, C. Maximino, et al., “Infl uence of gender and estrous cycle in the forced swim test in rats,” Psychol. Neurosci., 1, No. 2, 191–197 (2008).
E. Grauer and Y. Kapon, “Wistar-Kyoto rats in the Morris water maze, impaired working memory and hyper-reactivity to stress,” Behav. Brain Res., 59, 147–151 (1993).
T. Hoh and D. Cain, “Fractionating the nonspatial pretraining effect in the water maze task,” Behav. Neurosci., 111, 1285–1291 (1997).
A. Kiss, A. M. Delattre, S. Pereira, et al., “17β-Estradiol replacement in young, adult and intermediate-aged female ovariectomized rats promotes improvement of spatial reference memory and an antidepressant effect and alters monoamines and BDNF levels in memory- and depression-related brain areas,” Behav. Brain Res., 227, 100–108 (2012).
Li Liu, J. Ding, C. Marshall, et al., “Pretraining affects Morris water maze performance with different patterns between control and ovariectomized plus d-galactose-injected mice,” Behav. Brain Res., 217, 244–247 (2011).
M. Lindner and T. Schallert, “Aging and atropine effects on spatial navigation in the Morris water task,” Behav. Neurosci., 102, 621–634 (1988).
M. Lindner, “Reliability, distribution, and validity of age-related cognitive deficits in the Morris water maze,” Neurobiol. Learn. Mem., 68, No. 3, 203–220 (1997).
H. Meziane, A.-M. Ouagazza, L. Aubert, et al., “Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies,” Genes Brain Behav., 6, No. 2, 192–200 (2007).
R. Morris, “Spatial localization does not require the presence of local cues,” Learn. Motiv., 12, No. 2, 239–260 (1981).
R. Morris, “Development of a water maze procedure for studying spatial learning in the rat,” J. Neurosci. Meth., 11, 47–60 (1984).
M. Packard, J. Kohlmaier, and G. Alexander, “Posttraining intrahippocampal estradiol injections enhance spatial memory in male rats: interaction with cholinergic systems,” Behav. Neurosci., 110, 626–632 (1996).
M. Packard and L. Teather, “Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats,” NeuroReport, 8, 3009–3013 (1997).
H. Van Praag, T. Shubert, C. Zhao, and F. Gage, “Exercise enhances learning and hippocampal neurogenesis in aged mice,” J. Neurosci., 25, 8680–8685 (2005).
A. Rissanen, J. Puolivali, T. van Groen, and P. Riekkinen, Jr., “In mice tonic estrogen replacement therapy improves non-spatial and spatial memory in a water maze task,” NeuroReport, 10, 1369–1372 (1999).
E. Rissman, S. Wersinger, H. Fugger, and T. Foster, “Sex with knock out models: behavioral studies of estrogen receptor alpha,” Brain Res., 835, 80–90 (1999).
R. Roof, “Neonatal exogenous testosterone modifies sex difference in radial arm and Morris water maze performance in prepubescent and adult rats,” Behav. Brain Res., 53, 1–10 (1993).
S. Royle, F. Collins, H. Rupniak, et al., “Behavioural analysis and susceptibility to CNS injury of four inbred strains of mice,” Brain Res., 816, 337–349 (1999).
B. Saab, A. Saab, and J. Roder, “Statistical and theoretical considerations for the platform re-location water maze,” J. Neurosci. Meth., 198, 44–52 (2011).
D. Saucier, and D. Cain, “Spatial learning without NMDA receptor-dependent long-term potentiation,” Nature, 378, 186–189 (1995).
S. Shigeta, T. Misawa, T. Yoshida, et al., “Neurobehavioral analysis of high-rate Sidman avoidance rat strain,” Yakubutsu Seishin Kodo, 9, 217–224 (1989).
J. Simpson and J. Kelly, “The impact of environmental enrichment in laboratory rats – behavioural and neurochemical aspects,” Behav. Brain Res., 222, 246–264 (2011).
R. Stackman, M. Blasberg, C. Langan, and A. Clark, “Stability of spatial working memory across the estrous cycle of Long–Evans rats,” Neurobiol. Learn. Mem., 67, No. 2, 167–171 (1997).
A. Terry, “Spatial navigation (water maze) tasks,” in: Methods of Behavior Analysis in Neuroscience, J. J. Buccafusco (ed.), CRC Press, Boca Raton (2009), 2nd ed.
C. Vorhees and M. Williams, “Morris water maze: procedures for assessing spatial and related forms of learning and memory,” Nat. Protoc., 1, No. 2, 848–858 (2006).
M. Waller, P. Waller, and L. Brewster, “A water maze for use in studies of drive and learning,” Psychol. Rep., 7, 99–102 (1960).
S. Warren and J. Juraska, “Spatial and nonspatial learning across the rat estrous cycle,” Behav. Neurosci., 111, No. 2, 259–266 (1997).
I. Whishaw and J.-A. Tomie, “Of mice and mazes: similarities between mice and rats on dry land but not water maze,” Physiol. Behav., 60, 1191–1197 (1996).
D. Wolfer, M. Stagljar-Bozicevic, M. Errington, and H.-P. Lipp, “Spatial memory and learning in transgenic mice: fact or artifact?” Physiology, 13, No. 3, 118–123 (1998).
D. Wolfer, R. Madani, P. Valenti, and H. Lipp, “Extended analysis of path data from mutant mice using the public domain software Wintrack,” Physiol. Behav., 73, 745–753 (2001).
P. Woods, E. Davidson, and R. Peters, “Instrumental escape conditioning in a water tank: effects of variation in drive stimulus intensity and reinforcement magnitude,” J. Comp. Psychol., 57, 466–470 (1964).
J. Yau, K. McNair, J. Noble, et al., “Enhanced hippocampal long-term potentiation and spatial learning in aged 11-hydroxysteroid dehydrogenase type 1 knock-out mice,” J. Neurosci., 27, 10487–10496 (2007).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 102, No. 1, pp. 3–17, January, 2016.
Rights and permissions
About this article
Cite this article
Ivlieva, A.L., Petritskaya, E.N., Rogatkin, D.A. et al. Methodological Characteristics of the Use of the Morris Water Maze for Assessment of Cognitive Functions in Animals. Neurosci Behav Physi 47, 484–493 (2017). https://doi.org/10.1007/s11055-017-0425-z
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-017-0425-z