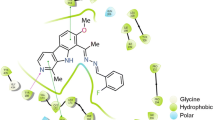
Hopantenic acid is a known nootropic agent with a chemical structure close to that of pantothenic acid (vitamin B5). The neurotropic effect of hopantenic acid may occur as a result of binding with δ and κ opioid receptors, modulating acetylcholine secretion, and interacting with dopamine receptors. Apart from the neurotropic effects, hopantenic acid can modulate the metabolism of prostaglandins and steroids, and may have antitumor actions.
Similar content being viewed by others
References
Hopantenic Acid. Encylopedia of Drugs of the Russian Drug Register, Moscow (2014).
S. Noda, J. Haratake, A. Sasaki, et al., “Acute encephalopathy with hepatic steatosis induced by pantothenic acid antagonist, calcium hopantenate, in dogs,” Liver, 11, No. 3, 134–142 (1991).
I. Yu. Torshin and K. V. Rudakov, “On the application of the combinatorial theory of solvability to the analysis of chemographs. Part 1: Fundamentals of modern chemical bonding theory and the concept of the chemograph,” Pattern Recognition and Image Analysis, 24, No. 1, 11–23 (2014).
I. Yu. Torshin and K. V. Rudakov, “On the application of the combinatorial theory of solvability to the analysis of chemographs: Part 2. Local completeness of invariants of chemographs in view of the combinatorial theory of solvability,” Pattern Recognition and Image Analysis., 24, No. 2, 196–208 (2014).
I. Yu. Torshin and O. A. Gromova, Expert Analysis of Data in Molecular Pharmacology, Moscow Center for Continuing Mathematical Education Press, Moscow (2012).
K. V. Rudakov and I. Yu Torshin, “Analysis of the informativeness of motifs based on the solvability criterion in the recognition of protein secondary structure,” Informat. Primen., 5, No. 4, 40–50 (2011).
Yu. I. Zhuravlev, K. V. Rudakov, and I. Yu. Torshin, “Algebraic criteria for local solvability and regularity as a tool for studying the morphology of amino acid sequences,” Tr. MFTI, 3, No. 4, 67–76 (2011).
K. V. Rudakov and I. Yu. Torshin, “Selection of informative values of parameters based on solvability criteria in the recognition of protein secondary structure,” Dokl. Akad. Nauk., 441, No. 1, 1–5 (2011).
I. Yu. Torshin, “On solvability, regularity, and locality of the problem of genome annotation,” Pattern Recognition and Image Analysis, 20, No. 3, 386–395 (2010).
Yu. I. Zhuravlev, “Set theory methods in the algebra of logic,” Probl. Kibernet., 8, No. 1, 25–45 (1962).
Yu. I. Zhuravlev, “Correct algebras over sets of incorrect (heuristic) algorithms,” Kibernetika, 4, 5–17 (1977).
Yu. I. Zhuravlev, “Algebraic approaches to solving the tasks of recognition and classifi cation,” in: Problems in Cybernetics, Nauka, Moscow (1978), Iss. 33, pp. 5–68.
Y. Wang, T. Suzek, J. Zhang, et al., “PubChem BioAssay: 2014 update,” Nucl. Acids Res., 42 (Database issue), D1075-1082 (2014).
D. S. Wishart, T. Jewison, A. C. Guo, et al., “HMDB 3.0 – The Human Metabolome Database in 2013,” Nucl. Acids Res., 41, D801-807 (2013).
S. Dutta, D. Dimitropoulos, Z. Feng, et al., “Improving the representation of peptide-like inhibitor and antibiotic molecules in the Protein Data Bank,” Biopolymers, 101, No. 6, 659–668 (2014).
T. A. Voronina, Pantogam and Pantogam Active. Pharmacological Effects and Mechanisms of Action, Research Institute of Pharmacology, Russian Academy of Medical Sciences, Moscow.
A. M. Ovezov, M. A. Lobov, M. V. Panteleeva, et al., “Correction of early cognitive disorders in school-age children operated under total intravenous anaesthesia,” Anestez. Reanimatol., No. 3, 25–29 (2012).
A. D. Corbett, G. Henderson, A. T. McKnight, and S. J. Paterson, “75 years of opioid research: the exciting but vain quest for the Holy Grail,” Br. J. Pharmacol., 147, Supplement, 153–162 (2006).
M. Waldhoer, S. E. Bartlett, and J. L. Whistler, “Opioid receptors,” Ann. Rev. Biochem., 73, 953–990 (2004).
B. N. Dhawan, F. Cesselin, R. Raghubir, et al., “International Union of Pharmacology. XII. Classifi cation of opioid receptors,” Pharmacol. Rev., 48, No. 4, 567–592 (1996).
A. Janecka, J. Fichna, and T. Janecki, “Opioid receptors and their ligands, Curr. Top. Med. Chem., 4, No. 1, 1–17 (2004).
B. Katzung, Basic and Clinical Pharmacology, McGraw-Hill Medical (2007), 10th ed., pp. 489–490, p. 1200.
E. V. Varga, E. Navratilova, D. Stropova, et al., “Agonist-specific regulation of the delta-opioid receptor,” Life Sci., 76, No. 6, 599–612 (2004).
R. M. Quock, T. H. Burkey, E. Varga, et al., “The delta-opioid receptor: molecular pharmacology, signal transduction, and the determination of drug efficacy,” Pharmacol. Rev., 51, No. 3, 503–532 (1999).
J. Zhang, H. Qian, P. Zhao, et al., “Rapid hypoxia preconditioning protects cortical neurons from glutamate toxicity through delta-opioid receptor,” Stroke, 37, No. 4, 1094–1099 (2006).
L. Guo, L. Zhang, and D. C. Zhang, “Mechanisms of delta-opioids cardioprotective effects in ischemia and its potential clinical applications,” Sheng Li Ke Xue Jin Zhan, 36, No. 4, 333–336 (2005).
Z. Z. Pan, “mu-Opposing actions of the kappa-opioid receptor,” Trends Pharmacol. Sci., 19, No. 3, 94–98 (1998).
K. Yamada, M. Imai, and S. Yoshida, “Mechanism of diuretic action of U-62,066E, a kappa opioid receptor agonist,” Eur. J. Pharmacol., 160, No. 2, 229–237 (1989).
E. Zeynalov, M. Nemoto, P. D. Hurn, et al., “Neuroprotective effect of selective kappa opioid receptor agonist is gender specific and linked to reduced neuronal nitric oxide,” J. Cereb. Blood Flow Metab., 26, No. 3, 414–420 (2006).
O. A. Gromova, I. Yu. Torshin, T. R. Grishina, et al., “Systematic analysis of the molecular-physiological effects myoinositol: molecular biology data and experimental and clinical medicine,” Effektiv. Farmakoter, 28, 4–12 (2013).
O. A. Gromova, E. A. Goncharova, I. Yu. Torshin, et al., “Potential for the use of myoinositol in the pregravid preparation of women with polycystic ovaries and insulin resistance,” Ginekologiya, No. 1, 58–65 (2014).
O. A. Gromova, I. Yu. Torshin, and O. A. Limanova, “Potential for the use of myoinositol in women with polycystic ovaries and insulin resistance in programs for the pregravid preparation for in vitro fertilization,” Akusher. Ginekol., No. 5, 12–23 (2013).
Rang & Dale’s Pharmacology, Churchill Livingstone (2011).
U. Yokoyama, K. Iwatsubo, M. Umemura, et al., “The prostanoid EP4 receptor and its signaling pathway,” Pharmacol. Rev., 65, No. 3, 1010–1052 (2013).
M. V. Karlina, O. N. Pozharitskaya, V. M. Kosman, et al., “Studies of the pharmacokinetics of hopantenic acid after oral administration,” Eksperim. Klin. Farmakol., 73, No. 8, 46–48 (2010).
L. Dong, J. Wen, E. Pier, et al., “Melanocyte-stimulating hormone directly enhances UV-Induced DNA repair in keratinocytes by a xeroderma pigmentosum group A-dependent mechanism,” Cancer Res., 70, No. 9, 3547–3556 (2010).
S. K. Manna and B. B. Aggarwal, “Alpha-melanocyte-stimulating hormone inhibits the nuclear transcription factor NF-kappaB activation induced by various infl ammatory agents,” J. Immunol., 161, No. 6, 2873–2880 (1998).
A. L. Kadekaro, J. Chen, J. Yang, et al., “Alpha-melanocyte-stimulating hormone suppresses oxidative stress through a p53-mediated signaling pathway in human melanocytes,” Mol. Cancer Res., 10, No. 6, 778–786 (2012).
G. W. Millington, “Proopiomelanocortin (POMC): the cutaneous roles of its melanocortin products and receptors,” Clin. Exp. Dermatol., 31, No. 3, 407–412 (2006).
G. W. Millington, “The role of proopiomelanocortin (POMC) neurones in feeding behaviour,” Nutr. Metab., 4, 18 (2007).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Nevrologii i Psikhiatrii imeni S. S. Korsakova, Vol. 115, No. 5, Iss 2, Pediatric Neurology and Psychiatry, pp. 61–71, May, 2015.
Rights and permissions
About this article
Cite this article
Gromova, O.A., Torshin, I.Y., Limanova, O.A. et al. The Neurotropic, Anti-Inflammatory, and Antitumor Properties of the Hopantenic Acid Molecule Based on Chemoinformatic Analysis. Neurosci Behav Physi 46, 1097–1106 (2016). https://doi.org/10.1007/s11055-016-0357-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-016-0357-z