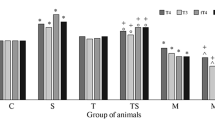
Treatment of rats with mercazolyl (25 mg/kg for 20 days), which decreases blood levels of iodine-containing thyroid hormones (ITH), decreased trypsin-like activity (TLA) and α1-antitrypsin (α1-AT) and α2-macroglobulin (α2-MG) activities in the liver and blood; in the stage of anxiety associated with the stress reaction (1 h after swimming in a vessel for 1 h), experimental rats showed more marked stimulation of proteolysis than seen in euthyroid animals, due to decreases in α1-AT and α2-MG activities, while in the resistance stage (at 48 h), the normalization of TLA and α1-AT and α2-MG levels occurring in stressed euthyroid rats was blocked; in the exhaustion stage (stressing for 1 h for 10 days), there was greater activation of proteolysis in experimental rats due to profound suppression of α1-AT and α2-MG activities. Administration of L-thyroxine (1.5–3.0 μg/kg for 28 days, which did not alter the blood ITH concentration, had no effect on the proteolysis system; in the anxiety and exhaustion stages, the increase in TLA was limited, while in the resistance stage this was prevented, with elimination of the depression of α1-AT and α2-MG activities. These results demonstrated a novel aspect of the involvement of ITH in the body’s antistress system, i.e., their influences on the proteinase/inhibitors system.
Similar content being viewed by others
References
S. N. Bondarenko, N. A. Bondarenko, and E. B. Manukhina, “Effects of different methods of stressing and adaptation to behavioral and somatic parameters in rats,” Byull. Eksperim. Biol. Med., 128, No. 8, 157–160 (1999).
K. N. Veremeenko, P. P. Goloborod’ko, and A. I. Kizim, Proteolysis in Health and Pathology, Zdorov’ya, Kiev (1988).
A. F. Vismont and L. M. Lobanok, “The involvement of liver arginase in detoxification and thermoregulation processes in endotoxin fever,” Voenn. Med. No. 1, 105–109 (2011).
Yu. A. Vladimirov and A. I. Archakov, Lipid Peroxidation in Biological Membranes, Nauka, Moscow (1972).
V. R. Ibragimov, V. N. Kozlov, Yu. V. Kas’yanova, and G. R. Timerbulatova, “Effects of thyrostatic agents on the histological structure of the liver in experimental studies in rats,” Pratsi TDATU, 2, No. 12, 141–146 (2010).
I. Yu. Karyagina, R. A. Zarembskii, and M. D. Balyabina, “Use of a complex method to measure the activity of trypsin-like proteases, α1-antitrypsin, and α2-macroglobulin in the gastroenterology clinic,” Lab. Delo, No. 2, 10–13 (1990).
N. G. Makarova, L. S. Vasil’eva, and D. V. Garmaeva, “Liver structure in experimental hypothyroidism,” Sib. Med. Zh., 93, No. 2, 42–44 (2010).
I. Yu. Malyshev, L. Yu. Golubeva, A. P. Bozhko, and I. V. Gorodetskii, “The role of local stress-limiting systems in the myocardium in the protective cardiac effect of low doses of thyroid hormones in restraint stress in rats,” Ros. Fiziol. Zh., 86, No. 1, 62–67 (2000).
D. K. Mardas, V. N. Nikandrov, et al., “The role of m-cholinoreceptors in controlling the balance of the proteolysis in heat stress,” in: Functional Systems of the Body in Health and Pathology, RICVSh, Minsk (2008), pp. 147–150.
N. L. Rendakov, “Changes in the activity of proteolytic activity of lysosomes on exposure to mercazolyl and thyroxine in foxes,” Vestn. Molod. Uchen. Ser. Nauk. Zhizn. No. 1, 61–67 (2004).
T. G. Sazonova and A. A. Matskevich, “Tissue specificity of the protective actions of cytoplasmic factors in a membrane-bound Ca2+ transport system in the sarcoplasmic reticulum of the heart and skeletal muscle,” Patol. Fiziol. Eksperim. Terr., 2, 3–6 (2000).
A. F. Ajayi and R. E. Akhigbe, “Implication of altered thyroid state on liver function,” Thyroid Res. Pract., 9, 84–87 (2012).
G. Capasso, G. De Tommaso, A. Pica, et al., “Effects of thyroid hormones on heart and kidney functions,” Miner. Electrolyte Metab., 25, No. 1–2, 56–64 (1999).
V. I. Chernaia, “Effect of one-time and chronic low-intensity irradiation on cathepsin L activity in rat brain,” Ukr. Biokhim. Zh., 73, No. 2, 97–101 (2001).
G. Fuhrmann, E. Kempf, and A. Ebel, “Effects of hormone therapy on the central cholinergic neurotransmission of the Snell dwarf mouse,” J. Neurosci. Res., 16, No. 3, 527–539 (1986).
R. Gredilla, M. López Torres, M. Portero-Otín, et al., “Influence of hyper- and hypothyroidsm on lipid peroxidation, unsaturation of phospholipids, glutathione system and oxidative damage to nuclear and mitochondrial DNA in mice skeletal muscle,” Mol. Cell. Biochem., 221, No. 1–2, 41–48 (2001).
M. E. Harper and E. L. Seifert, “Thyroid hormone effects on mitochondrial energetics,” Thyroid, 18, No. 2, 145–156 (2008).
B. Kim, S. D. Carvalho-Bianco, and P. R. Larsen, “Thyroid hormone and adrenergic signaling in the heart,” Arq. Bras. Endocrinol. Metab., 48, No. 1, 171–175 (2004).
O. H. Lowry, N. J. Rosebrough, A. L. Farr, and R. J. Randall, “Protein measurement with the Folin phenol reagent,” J. Biol. Chem., 193, No. 1, 265–275 (1951).
A. Negre-Salvayre, C. Coatrieux, C. Ingueneau, and R. Salvayre, “Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors,” Br. J. Pharmacol., 153, No. 1, 6–20 (2008).
D. Neil, N. D. Rawlings, and G. Salvesen, Handbook of Proteolytic Enzymes, Academic Press, Oxford (2013).
X. G. Zhu, P. McPhie, K. H. Lin, and S. Y. Cheng, “The differential hormone-dependent transcriptional activation of thyroid hormone receptor isoforms is mediated by interplay of their domains,” J. Biol. Chem., 272, No. 14, 9048–9054 (1997).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 99, No. 12, pp. 1378–1388, December, 2013.
Rights and permissions
About this article
Cite this article
Gorodetskaya, I.V., Gusakova, E.A. The Effects of Thyroid Status on the Proteolysis System in Stress. Neurosci Behav Physi 45, 693–700 (2015). https://doi.org/10.1007/s11055-015-0130-8
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-015-0130-8