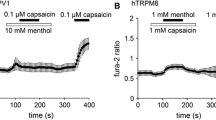
Animal experiments were performed to study the effects of activation of cold- and menthol-sensitive TRPM8 receptors by menthol on thermoregulatory parameters in thermoneutral conditions and on cooling. Total oxygen consumption was measured, along with expiration of carbon dioxide, the respiratory coefficient, the skin vessel constriction reaction, and contractile muscle activity. Application of menthol in thermoneutral conditions led to increased oxygen consumption and a decrease in the respiratory coefficient which, in the absence of shivering, provides evidence of increased non-contractile thermogenesis and lipolysis. Cooling on the background of activation of the TRPM8 receptor was characterized by decreases in the thresholds of all thermoregulatory reactions with no change in their initiation sequence, along with increases in the metabolic components of emergency thermogenesis, leading to improved maintenance of deep body temperature when environmental cold acts on the body. These results provide evidence for a contribution of TRPM8 ion channels to increased activation of thermoreceptor structures generating afferent signals involved in maintaining metabolism in thermoneutral conditions and forming the structure of the efferent response of the body to cold.
Similar content being viewed by others
References
T. V. Kozyreva, “Modulation of the functional properties of cutaneous thermoreceptors,” Neirofiziologiya, 24, No. 5, 542–552 (1992).
T. V. Kozyreva and L. A. Verkhoglyad, “Functional significance of the dynamic activity of cutaneous cold receptors,” Fiziol. Zh. SSSR, 75, No. 1, 117–123 (1989).
T. V. Kozyreva and L. A. Verkhoglyad, “Cold adaptation and the structure of the thermoregulatory response in slow and rapid cooling,” Ros. Fiziol. Zh. im. I. M. Sechenova, 83, No. 11–12, 135–142 (1997).
T. V. Kozyreva and E. Ya. Tkachenko, “Effects of menthol on temperature sensitivity in humans, Fiziol. Cheloveka, 34, No. 2, 57–62 (2008).
E. Ya. Tkachenko, V. P. Kozaruk, and R. V. Kozyreva, “Effects of modulation of cutaneous thermoreceptors by capsaicin on various measures of thermoregulation in the warm and on cooling,” Byull. Sib. Otd. Ros. Akad. Med. Nauk, 1, 119–122 (2001).
M. C. Almeida, A. A. Steiner, L. G. S. Branco, and A. A. Romanovsky, “Cold behavior as thermoregulatory strategy in systemic inflammation,” Eur. J. Neurosci., 23, 3359–3367 (2006).
C. Cabanes, F. Viana, and C. Belmonte, “Differential thermosensitivity of sensory neurons in the guinea pig trigeminal ganglion,” J. Neurophysiol., 90, 2219–2231 (2003).
H. Chuang, E. Prescott, H. Kong, S. Shields, S. Jordt, A. Basbaum, M. Chao, and D. Julius, “Bradykinin and nerve growth factor release the capsaicin receptor from PtdInst(4,5)P2-mediated inhibition,” Nature, 411, 957–962 (2001).
H. Chuang, W. Neuhausser, and D. Julius, “The super cooling agent icilin reveals a mechanism of coincidence detection by a temperature-sensitive TRP channel,” Neuron, 43, 859–869 (2004).
A. Dhaka, T. J. Earley, J. Watson, and A. Patapoutian, “Visualizing cold spots: TRPM8-expressing sensory neurons and their projections,” J. Neurosci., 28, No. 3, 566–575 (2008).
S. N. Davis, G. E. Goldsmith, R. F. Hellon, and D. Mitchell, “Facial sensitivity to rates of temperature change: neurophysiological psychological evidence from cats and human, “ J. Physiol., 344, No. 1, 135–146 (1983).
S. Jordt, D. McKemy, and D. Julius, “Lessons from peppers and peppermint: the molecular logic of thermoregulation,” Curr. Opin. Neurobiol., 13, No. 1, 1–6 (2003).
E. Kandel, J. Schwartz, and T. Jessell, Principles of Neural Science, McGraw-Hill, New York (2000).
Y. Karashima, N. Damann, J. Prenen, K. Talavera, A. Segal, T. Voets, and B. Nilius, “Bimodal action of menthol on the transient receptor potential channel TRPA1,” J. Neurosci., 27, No. 37, 9874–9884 (2007).
K. Kobayashi, T. Fukuoka, K. Obata, H. Yamanaka, Y. Dai, A. Tokunaga, and K. Noguchi, “Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors,” J. Comp. Neurol., 493, 596–606 (2005).
T. V. Kozyreva, E. Ya. Tkachenko, and V. P. Kozaruk, “Thermoregulatory responses to cooling before and after the noradrenaline iontophoresis to skin,” J. Therm. Biol., 24, No. 1, 175–183 (1999).
L. J. Macpherson, A. E. Dubin, M. J. Evans, F. Marr, P. G. Schulz, B. F. Cravatt, and A. Patapoutian, “Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines.” Nature, 445, 541–545 (2007).
Yu. Masamoto, F. Kawabata, and T. Fushiki, “Intragastric administration of TRPV1, TRPV3, TRPM8, and TRPA1 agonists modulates autonomic thermoregulation in different manners in mice,” Biosci. Biotechnol. Biochem., 73, No. 5, 1021–1027 (1990).
D. McKemy, “How cold is it? TRPM3 and TRPA1 in the molecular logic of cold,” Mol. Pain., No. 1, 1–16 (2005).
D. McKemy,W. Neuhausser, and D. Julius, “Identification of a cold receptor reveals a general role for TRP channels in thermosensation,” Nature, 416, 52–58 (2002).
I. B. Mekjavic, A. La Prairie, A. Burke, and B. Lindborg, “Respiratory drive during sudden cold water immersion,” Respir. Physiol., 70, No. 1, 121–130 (1987).
A. Patapoutian, A. Peier, G. Story, and V. Vismanath, “ThermoTRP channels and beyond: mechanisms of temperature sensation,” Neurosci., 4, 529–539 (2003).
A. Peier, A. Mogrich, A. Hergarden, A. Reeve, D. Andersson, G. Story, T. Earley, I. Gragoni, P. McIntyre, S. Bevan, and A. Patapoutian, “A TRP channel that senses cold stimuli and menthol,” Cell, 108, 705–715 (2002).
G. Reid and M. Flonta, “Cold transduction by inhibition of a background potassium conductance in rat primary sensory neurons,” Neurosci. Lett., 297, 171–174 (2001).
G. Reid and M. Flonta, “Physiology: Cold current in thermoreceptive neurons,” Nature, 413, 480–485 (2001).
G. M. Story, A. M. Peir,A. J. Reeve, S. R. Eid, J. Mosbacher, T. R. Hricik, T. J. Earley, A. C. Hergarde, D. A. Andersson, S. W. Hwang, P. McIntyre, T. Jegla, S. Bevan, and A. Patapoutian, “ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures,” Cell, 112, 819–829 (2003).
K. Suto and H. Gotoh, “Calcium signaling in cold cells studied in cultured dorsal root ganglion neurons,” Neurosci., 92, 1131–1135 (1999).
K. Tajino, K. Matsumara, K. Kosada, T. Shibakusa, K. Inoue, T. Fushuki, H. Hosokawa, and S. Kobayashi, “Application of menthol to the skin of whole trunk in mice induces autonomic and behavioral heat-gain responses,” Am. J. Physiol. Regul. Integr. Comp. Physiol., 293, R2128–R2135 (2007).
P. Thut, D. Wrigley, and M. Gold, “Cold transduction in rat trigeminal ganglia neurons in vitro,” Neurosci., 119, 1071–1083 (2003).
E. Ya. Tkachenko, S. V. Lomakina, and T. V. Kozyreva, “Modulating effect of calcium on the cold defence response formation in normotensive and hypertensive rats,” J. Therm. Biol., 30, No. 7, 545–550 (2005).
F. Viana, E. de la Pena, and C. Belmonte, “Specificity of cold thermotransduction is determined by differential ionic channel expression,” Nature Neurosci., 5, 254–260 (2002).
B. Xiao, A. Dubin, B. Bursulaya,V. Viswanath, T. Jegla, and A. Patapoutian, “Identification of the transmembrane domain five as a critical molecular determinant of menthol sensitivity in mammalian TRPA1 channels,” J. Neurosci., 28, No. 39, 9640–9651 (2008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 97, No. 2, pp. 218–226, February, 2011.
Rights and permissions
About this article
Cite this article
Kozyreva, T.V., Kozaruk, V.P., Tkachenko, E.Y. et al. Effects of Activation of TRPM8 Ion Channels on Thermoregulatory Reactions in Cooling. Neurosci Behav Physi 42, 654–659 (2012). https://doi.org/10.1007/s11055-012-9617-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-012-9617-8