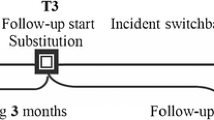
Quantitative analysis of the causes of termination of pharmaceutical remission lasting more than one year was undertaken in 220 adult patients with epilepsy. The most frequent cause of loss of seizure control was switching from an original proprietary medicine to a generic analog (60.4 %); a total of 28.2 % of patients had been switched to generic topiramate. Comparative analysis of the results of switching of 160 patients form the original form of topiramate (Topamax) to its generics was performed. The control group consisted of 52 patients continuing the original formulation. Switching led to loss of remission in 75.6 % of patients, with status epilepticus in 3.75 % and emergency care or hospitalization were required in 51.9 % of patients. Switching back to the original formulation was performed in 86.2 % of patients, after which the initial doses of Topamax were increased in 58.0 %, 60.0 % of patients transferred from monotherapy to polytherapy, and baseline levels of seizure control were achieved in only 32.9 % of patients.
Similar content being viewed by others
References
A. P. Meshkovskii, “The place of generics in drug treatment,” Farmateka, 3, 103–104 (2003).
S. M. Kharchuk, A. B. Gekht, E. D. Belousova, et al., “Rational pharmacotherapy of epilepsy: traditional and new approaches to overcoming old problems,” Remedium, 11, No. 6, 16–17 (2007).
A. K. Basak, A. S. Raw, A. H. Hakim, et al., “Pharmaceutical impurities: regulatory perspective for Abbreviated New Drug Interactions,” Adv. Drug Deliv. Res., 59, 64–72 (2007).
M. J. Berg, R. A. Gross, K. J. Tomaszewski, et al., “Generic substitution in the treatment of epilepsy: case evidence of breakthrough seizures,” Neurology, 71, 525–530 (2008).
F. M. Besag, “Is generic prescribing acceptable in epilepsy?” Drug. Saf., 23, 173–182 (2000).
D. J. Birkett, “Generics – equal or not?” Austr. Prescr., 26, 85–87 (2003).
G. Borgherini, “The bioequivalence and therapeutic efficacy of generic versus brand-name psychoactive drugs,” Clin. Ther., 25, 1578–1592 (2003).
R. T. Burkhardt, I. E. Leppik, K. Blesi, et al., “Lower phenytoin serum levels in persons switched from brand to generic phenytoin,” Neurology, 63, 1494–1496 (2004).
M. L. Chen, V. Shah, R. Patnaik, et al., “Bioavailability and bioequivalence: an FDA regulatory overview,” Pharm. Res., 18, 1645–1650 (2001).
P. Crawford, M. Feely, and G. Kramer, “Are there potential problems with generic substitution of antiepileptic drugs? A review of issues,” Seizure, 15, 165–176 (2006).
R. Donnelly, P. A. Meredith, S. H. Miller, et al., “Pharmacodynamic modeling of the antihypertensive response to amlodipine,” Clin. Pharmacol. Ther., 54, 303–310 (1993).
M. S. Duh, P. E. Paradis, D. Latremouille-Viau, et al., “The risks and costs of multiple-generic substitution of topiramate,” Neurology, 72, 2122–2129 (2009).
European Medicines Agency, Committee for Proprietary Medicinal Products, Note for Duidance on the Investigation of Bioavailability and Bioequivalence, CPMP/EWP/QWP/1401/98, July 2001.
J. A. French and B. E. Gidal, “Antiepileptic drug interactions,” Epilepsia, 41, Suppl. 8, 30–36 (2000).
B. D. Furgerg and C. D. Furgerg, “Are all drugs of a class interchangeable?” in: Evaluating Clinical Research: All That Glitters Is Not Gold, Springer, New York (2007), pp. 115–119.
A. A. Genazzani and F. Pattarino, “Difficulties in the production of identical drug products from a pharmaceutical technology viewpoint,” Drugs R. D., 9, 65–72 (2008).
G. Kramer, A. Biraben, M. Carreno, et al., “Current approaches to the use of generic antiepileptic drugs,” Epilepsy and Behavior, 11, 46–52 (2007).
P. Meredith, “Bioequivalence and other unresolved issues in generic drug substitution,” Clin. Ther., 25, 2575–2590 (2003).
G. F. Meyer, “History and regulatory issues of generic drugs,” Transplant. Proc., 31, Suppl. 3A, 10–12 (1999).
M. C. Meyer, A. B. Straughn, R. M. Mhatre, et al., “The relative bioavailability and in vivo-in vitro correlations for four marketed carbamazepine tablets,” Pharm. Res., 15, 1787–1791 (1998).
K. Nakai, M. Fujita, and H. Ogata, “New bioequivalence studies: individual bioequivalence and population bioequivalence,” Yakugaku Zasshei, 120, 1201–1208 (2000).
K. Nagai, M. Fujita, and H. Ogata, “International harmonization of bioequivalence studies and issues shared in common,” Yakugaku Zasshei, 120, 1193–1200 (2000).
M. Olling, T. T. Mensinga, D. M. Barends, et al., “Bioavailability of carbamazepine from four different products and the occurrence of side effects,” Biopharm. Drug. Dispos., 20, 19–28 (1999).
D. H. Rosenbaum, A. J. Rowan, L. Tuchman, and J. A. French, “Comparative bioavailability of a generic phenytoin and Dilantin,” Epilepsia, 35, 656–660 (1994).
T. Scott, E. W. Devine, J. Barron, and A. Behm, “Original article. Acute epilepsy exacerbation in patients switched between A-rated anti-epileptic drugs,” Curr. Med. Res. Opin., 26, No. 2, 455–463 (2010).
A. Wiecek and A. Mikhail, “European regulatory guidelines for biosimilars,” Nephrol. Dial. Transplant., 21, Supplement 5, 17–20 (2006).
A. N. Wilner, “Therapeutic equivalency of generic antiepileptic drugs: results of a survey,” Epilepsy Behav., 5, 995–998 (2004).
World Health Organization, www.who.int/trade/glossary/story034/en/index.html.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Nevrologii i Psikhiatrii imeni S. S. Korsakova, Vol. 111, No. 3, pp. 38–43, March, 2011.
Rights and permissions
About this article
Cite this article
Rudakova, I.G., Kotov, A.S. & Belova, Y.A. Use of Generic Medicines in the Treatment of Epilepsy Using Topiramate as an Example. Neurosci Behav Physi 42, 550–555 (2012). https://doi.org/10.1007/s11055-012-9599-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-012-9599-6