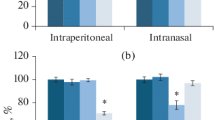
The heptapeptide Semax (MEHFPGP) is an analog of the fragment ACTH(4–10) with long-lasting actions. The aim of the present work was to study the effects of Semax on learning ability and pain sensitivity in white rats given different doses via the intraperitoneal and intranasal routes. The nootropic effects of Semax were studied in a test based on the acquisition of a conditioned passive avoidance reaction to pain stimulation. Pain sensitivity was assessed in a hindpaw compression test. The results showed that i.p. Semax had nootropic and analgesic actions. Dose-response characteristics were different for these different effects. Intranasal Semax was more effective in improving learning in animals than i.p. Semax but had no effect on pain sensitivity. Our results provide evidence that different mechanisms and brain structures are involved in mediating the nootropic and analgesic effects of Semax.
Similar content being viewed by others
References
I. P. Ashmarin, V. N. Nezavibatko, N. F. Myasoedov, A. A. Kamenskii, I. A. Grivennikov, M. A. Ponomareva-Stepnaya, L. A. Andreeva, A.Ya. Kaplan, V. B. Koshelev, and T. V. Ryasina, “A nootropic analog of adrenocorticotropic hormone 4–10 – Semax,” Zh. Vyssh. Nerv. Deyat., 47, No. 2, 420–430 (1997).
N. Yu. Glazova, E. A. Sebentsova, N. G. Levitskaya, L. A. Andreeva, L. Yu. Alfeeva, A. A. Kamenskii, and N. F. Myasoedov, “Effects of modification of the N-terminal part of the molecule on the extent of the nootropic action of Semax analogs,” Izv. Ros. Akad. Nauk. Ser. Biol., No. 4, 460–466 (2005).
D. M. Ivanova, N. G. Levitskaya, L. A. Andreeva, L. Yu. Alfeeva, A. A. Kamenskii, and N. F. Myasoedov, “Effects of Semax on pain sensitivity in animals in various experimental models,” Dokl. Ros. Akad. Nauk., 388, No. 3, 416–419 (2003).
D. M. Ivanova, D. A. Vilenskii, N. G. Levitskaya, L. A. Andreeva, L.Yu. Alfeeva, A. A. Kamenskii, and N. F. Myasoedov, “Studies of the relationship between the analgesic activity of melanocortin analogs and their structure,” Izv. Ros. Akad. Nauk. Ser. Biol., No. 2, 162–167 (2006).
A. A. Kamenskii, O. G. Voskresenskaya,V. A. Dubynin, and N. G. Levitskaya, “Relationship between the physiological effects of neuropeptides and the route of administration,” Ros. Fiziol. Zh. im. I. M. Sechenova, 87, No. 11, 1493–1501 (2001).
A. A. Kamenskii, N. Yu. Sarycheva, E. Yu. Baturina, and I. P. Ashmarin, “Intranasal administration of regulatory peptides,” Vestn. Akad. Med. Nauk. SSSR, No. 10, 43–47 (1988).
A. Ya. Kaplan,V. B. Koshelev,V. N. Nezavibatko, and I. P. Ashmarin, “Increases in the body’s resistance to hypoxia using the neuropeptide medicinal agent Semax,” Fiziol. Cheloveka, 18, No. 5, 104–107 (1992).
M. V. Koroleva, E. E. Meizerov,V. N. Nezavibatko, A. A. Kamenskii, V. A. Dubynin, and Y. B. Yakovlev, “Studies of the analgesic action of Semax,” Farmakol. Toksikol., 122, No. 11, 527–529 (1996).
N. G. Levitskaya, E. A. Sebentsova, L. A. Andreeva, L. Yu. Alfeeva, A. A. Kamenskii, and N. F. Myasoedov, “The neuroprotective effects of Semax on the background of MPTP-induced lesions to the brain dopaminergic system,” Ros. Fiziol. Zh. im. I. M. Sechenova, 88, No. 11, 1369–1377 (2002).
M. A. Ponomareva-Stepnaya, V. N. Nezavibatko, L. V. Antonomva, L. A. Andreeva, L. Yu. Alfeeva,V. N. Potaman, A. A. Kamenskii, and I. P. Ashmarin, “A long-acting ACTH4-10 analog which stimulates learning,” Khim.-Farm. Zh., 18, No. 7, 790–795 (1984).
I. P. Ashmarin, V. N. Nezavibatko, N. G. Levitskaya, V. B. Koshelev, and A. A. Kamensky, “Design and investigation of an ACTH(4–10) analogue lacking D-amino acids and hydrophobic radicals,” Neurosci. Res. Commun., 16, No. 2, 105–112 (1995).
J. Born, T. Lange,W. Kern, G. P. McGregor, U. Bickel, and H. L. Fehm, “Sniffing neuropeptides: a transnasal approach to the human brain,” Nat. Neurosci., 5, No. 6, 514–516 (2002).
X. Q. Chen, J. R. Fawcett,Y. E. Rahman, T. A. Ala, and W. H. Frey, “Delivery of nerve growth factor to the brain via the olfactory pathway,” J. Alzh. Dis., 1, No. 1, 35–44 (1998).
S. V. Dhuria, L. R. Hanson, and W. H. Frey, “Intranasal drug targeting of hypocretin-1 (orexin-A) to the central nervous system,” J. Pharm. Sci., 98, No. 7, 2501–2515 (2009).
S. V. Dhuria, L. R. Hanson, and W. H. Frey, “Intranasal delivery to the central nervous system: mechanisms and experimental considerations,” J. Pharm. Sci., 99, No. 4, 1654–1673 (2010).
O. V. Dolotov, E. A. Karpenko, L. S. Inozemtseva, T. S. Seredenina, N. G. Levitskaya, J. Rozyczka, E. V. Dubynina, E. V. Novosadova, L. A. Andreeva, L. Yu. Alfeeva, A. A. Kamensky, I. A. Grivennikov, N. F. Myasoedov, and J. Engele, “Semax, an analog of ACTH(4-10) with cognitive effects, regulates BDNF and trkB expression in the rat hippocampus,” Brain Res., 1117, No. 1, 54–60 (2006).
M. Fekete and D. De Wied, “Dose-related facilitation and inhibition of passive avoidance behaviour by the ACTH(4–10) analog (ORG 2766),” Pharmacol. Biochem. Behav., 17, No. 2, 177–182 (1982).
H. M. Greven and D. De Wied, “Influence of peptides structurally related to ACTH and MSH on active avoidance in rats. A structureactivity relationship study,” Front. Horm. Res., 4, 140–152 (1977).
L. R. Hanson and W. H. Frey, “Strategies for intranasal delivery of therapeutics for the prevention and treatment of neuroAIDS,” J. Neuroimmune Pharm., 2, 81–86 (2007).
L. R. Hanson and H. F. Frey, “Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease,” BMC Neurosci., 9, No. 3, S5 (2008).
A. A. Hussain, “Intranasal drug delivery,” Adv. Drug Deliv. Rev., 29, No. 1–2, 39–49 (1998).
L. Illum, “Transport of drugs from nasal cavity to central nervous system,” Eur. J. Pharm. Sci., 11, 1–18 (2000).
V. N. Potaman, L. V. Antonova,V. A. Dubynin, D. A. Zaitzev, A. A. Kamensky, N. F. Myasoedov, and V. N. Nezavibatko, “Entry of the synthetic ACTH(4-10) analogue into the rat brain following intravenous injection,” Neurosci. Lett., 127, No. 1, 133–138 (1991).
S. M. South and M. T. Smith, “Apparent sensitivity of the hotplate latency test for detection of antinociception following intraperitoneal, intravenous or intracerebroventricular M6G administration to rats,” J. Pharmacol. Exp. Ther., 286, No. 3, 1326–1332 (1998).
K. Atarowicz and B. Przewlocka, “The role of melanocortins and their receptors in inflammatory processes, nerve regeneration and nociception,” Life Sci., 73, No. 7, 823–847 (2003).
R. G. Thorne, C. R. Emory, T. A. Ala, and W. H. Frey, “Quantitative analysis of the olfactory pathway for drug delivery to the brain,” Brain Res., 692, No. 1–2, 278–282 (1995).
J. E. C. Wikberg, R. Muceniece, I. Mandrika, P. Prusis, J. Lindblom, C. Post, and A. Skottner, “New aspects on the melanocortins and their receptors,” Pharmacol. Res., 42, No. 5, 393–420 (2000).
J. E. Wikberg and F. Mutulis, “Targeting melanocortin receptors: an approach to treat weight disorders and sexual dysfunction,” Nature Rev. Drug Discov., 7, No. 4, 307–323 (2008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 96, No. 10, pp. 1014–1023, October, 2010.
Rights and permissions
About this article
Cite this article
Manchenko, D.M., Glazova, N.Y., Levitskaya, N.G. et al. The Nootropic and Analgesic Effects of Semax Given via Different Routes. Neurosci Behav Physi 42, 264–270 (2012). https://doi.org/10.1007/s11055-012-9562-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-012-9562-6