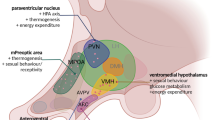
Orexin is a hypothalamic peptide neurotransmitter first described in 1998. Orexinergic neurons are located predominantly in hypothalamic structures and play an important role in regulating a variety of physiological functions. In the present article we review data on the locations of orexin-containing neurons in the brains of Wistar rats and their projections within the hypothalamus and to other CNS structures. The involvement of orexinergic neurons in regulating feeding behavior and sleep/waking cycles is reviewed, as is its involvement in controlling immune system functions. Experimental data providing a comparative analysis of the responses of orexin-containing neurons to antigenic and non-antigenic stimuli are presented, these providing evidence of the functional heterogeneity of hypothalamic orexin-containing neurons, which is particularly apparent in mediating the responses of the brain to antigenic and non-antigenic stimuli. Analysis of both changes in the intensity of the expression of the preproorexin gene and the morphofunctional characteristics of hypothalamic orexin-containing neurons after exposure to antigens provides grounds for their possible involvement in the mechanisms mediating the responses of the brains to antigens.
Similar content being viewed by others
References
P. K. Anokhin and K. V. Sudakov, “A neurophysiological theory of hunger, appetite, and satiation,” Usp. Fiziol. Nauk., 2, No. 1, 3 (1971).
Yu. V. Gavrilov, S. V. Perekrest, N. S. Novikova, and E. A. Korneva, “Stress-induced changes in the responses of cells in hypothalamic structures to administration of antigen (lipopolysaccharide) (in terms of c-Fos protein expression),” Ros. Fiziol. Zh. im. I. M. Sechenova, 92, No. 11, 1195–1203 (2006).
E. A. Korneva, T. B. Kazakova, and M. A. Nosov, “Expression of c-fos mRNA and c-Fos-like proteins in cells in hypothalamic structures after antigen administration,” Allergol. Immunol., 1, 37–44 (2001).
E. A. Korneva, V. M. Klimenko, and E. K. Shkhinek, Neurohumoral Support of Immune Homeostasis [in Russian], Nauka, Leningrad (1978).
E. A. Korneva and L. M. Khai, “Effects of lesioning areas of the hypothalamic region on immunogenesis,” Fiziol. Zh. SSSR, 49, No. 1, 42–48 (1963).
E. A. Korneva and E. K. Shkhinek, Hormones and the Immune System [in Russian], Nauka, Leningrad (1988).
A. I. Lakomkin and I. F. Myagkov, Hunger and Thirst [in Russian], Meditsina, Moscow (1975).
E. S. London, “Effects of removal of different parts of the brain on immunity to anthrax in pigeons,” Arkh. Biol. Nauk., 7, 177–187 (1899).
F. Z. Meerson and G. T. Sukhikh, “Stress-induced impairments in the antitumor immunity system and their restriction by stress-limiting factors,” Vestn. Akad. Med. Nauk. SSSR, 8, 23–29 (1985).
S. V. Perekrest, Yu. V. Gavrilov, N. S. Novikova, and E. A. Korneva, “Expression of c-Fos protein in cells in hypothalamic structures after administration of antigens of different natures,” Med. Immunol., 8, No. 2–3, 166–167 (2006).
S. V. Perekrest, N. S. Novikova, T. V. Abramova, Yu. V. Loskutov, V. J. Rodgers, and E. A. Korneva, “Patterns of activation in hypothalamic structures after administration of lipopolysaccharide at low and high doses,” Ros. Immunol. Zh., 2, No. 11, 156 (2008).
I. G. Savchenko, “On resistance to anthrax,” Vrach, 5, 132–134 (1891).
N. Alam, H. Gong, T. Alam, R. Jaganath, D. Ginty, and R. Szymusiak, “Sleep-waking discharge patterns of neurons recorded in the rat perifornical lateral hypothalamic area,” J. Physiol., 538, No. 2, 619–631 (2002).
K. A. Al-Barazanji, S. Wilson, J. A. Baker, D. S. Jessop, and M. S. Harbuz, “Central orexin-A activates hypothalamic-pituitary-adrenal axis and stimulates hypothalamic corticotropin releasing factor and arginine vasopressin neurones in conscious rats,” Neuroendocrinol., 13, 421–424 (2001).
V. R. Antunes, G. C. Brailoiu, E. H. Kwok, P. Scruggs, and N. J. Dunn, “Orexins/hypocretins excite rat sympathetic preganglionic neurons in vivo and in vitro,” Am. J. Physiol. Regul. Integr. Comp. Physiol., 281, 1801–1807 (2001).
J. A. Bartlett, M. K. Demetrikopoulos, and S. J. Schleifer, “Phagocytosis and killing of Staphylococcus aureus: effects of stress and depression in children,” Clin. Diagnostic Lab. Immunol., 4, No. 3, 362–366 (1997).
C. Becskei, N. Hernadfalvy, D. Arsenijevic, T. A. Lutz, W. Langhans, and T. Riediger, “Inhibitory effects of LPS on hypothalamic nuclei involved in the control of food intake,” Brain Behav. Immunol., 22, 56–64 (2007).
C. Becskei, T. Riediger, N. Hernadfalvy, D. Arsenijevic, T. A. Lutz, and W. Langhans, “Inhibitory effects of lipopolysaccharide on hypothalamic nuclei implicated in the control of food intake,” Brain Behav. Immunol., 22, No. 1, 56–64 (2008).
H. O. Besedovsky and A. Rey, “Immune-neuro-endocrine interactions: facts and hypotheses,” Endocrinol. Rev., 17, No. 1, 64–102 (1996).
S. Bingham, P. T. Davey, A. J. Babbs, E. A. Irving, M. J. Sammons, M. Wyles, P. Jeffrey, L. Cutler, I. Riba, A. Johns, R. A. Porter, N. Upton, A. J. Hunter, and A. A. Parsons, “Orexin-A, an hypothalamic peptide with analgesic properties,” Pain, 92, 81–90 (2001).
M. Blanco, M. Lopez, T. Garcia-Caballero, R. Gallego, A. Vazquez-Boquete, G. Morel, R. Señaris, F. Casanueva, C. Dieguez, and A. Beiras, “Cellular localization of orexin receptors in human pituitary,” J. Clin. Endocrinol. Metab., 86, 1616–1619 (2001).
K. Bulloch, B. S. McEwen, J. Nordberg, A. Diwa, and S. Baird, “Selective regulation of T-cell development and function by calcitonin gene-related peptide in thymus and spleen. An example of differential regional regulation of immunity by the neuroendocrine system,” Ann. N.Y. Acad. Sci., 840, 551–562 (1998).
K. Bulloch and W. Pomerantz, “Autonomic nervous system innervation of thymus-related lymphoid tissue in wild type and nude mice,” J. Comp. Neurol., 228, 57–68 (1984).
C. T. Chen, L. L. Hwang, J. K. Chang, and N. J. Dun, “Presser effects of orexins injected intracisternally and to rostral ventrolateral medulla of anesthetized rats,” Am. J. Physiol. Regul. Integr. Comp. Physiol., 278, 692–697 (2000).
T. R. Coppinge, J. E. Minton, and P. G. Reddy, “Repeated restraint and isolation stress in lambs increases pituitary-adrenal secretions and reduces cell-mediated immunity,” J. Anim. Sci., 69, 2808–2814 (1991).
Y. Date, M. S. Mondal, S. Matsukura, Y. Ueta, H. Yamashita, H. Kaiya, K. Kangawa, and M. Nakazato, “Distribution of orexin/hypocretin in the rat median eminence and pituitary,” Brain Res. Mol. Brain Res., 76, 1–6 (2000).
A. Denes, Z. Boldogkoi, G. Uhereczky, A. Hornyak, M. Rusvai, M. Palkovits, and K. J. Kovacs, “Central automatic control of the bone marrow: multisynaptic tract tracing by recombinant pseudorabies virus,” Neurosci., 134, No. 3, 947–963 (2005).
I. J. Elenkov, R. L. Wilder, G. P. Chrousos, and E. S. Vizi, “The sympathetic nerve – an integrative interface between two supersystems: the brain and the immune system,” Pharmacol. Rev., 52, No. 4, 595–638 (2000).
J. K. Elmquist and C. B. Saper, “Activation of neurons projecting to the paraventricular hypothalamic nucleus by intravenous lipopolysaccharide,” J. Comp. Neurol., 374, No. 3, 315–331 (1996).
M. Fleshner, T. K. Nguyen, C. S. Cotter, and L. R. Watkins, “Acute stressor exposure both suppresses acquired immunity and potentiates innate immunity,” Am. J. Physiol. Regul. Integr. Comp. Physiol., 282, R1680–R1686 (2002).
E. M. Friedman and D. A. Lawrence, “Environmental stress mediates changes in neuroimmunological interactions,” Toxicol. Sci., 67, 4–10 (2002).
R. P. A. Gayekema, L. E. Goehler, C. B. Armstrong, J. Khorsand, S. F. Maier, and L. R. Watkins, “Differential Fos expression rat brain induced by lipopolysaccharide and staphylococcal enterotoxin B,” Neuroimmunomodulation, 6, 220–226 (1999).
R. P. Gaykema, C. C. Chen, and L. E. Goehler, “Organization of immune-responsive medullary projections to the bed nucleus of the stria terminalis, central amygdale, and paraventricular nucleus of the hypothalamus: evidence for parallel viscerosensory pathways in the rat brain,” Brain Res., 1130, No. 1, 130–145 (2007).
R. P. Gaykema, L. E. Goehler, F. J. Tilders, J. G. Bol, M. McGorry, M. Fleshner, S. F. Maier, and L. R. Watkins, “Bacterial endotoxin induces Fos immunoreactivity in primary afferent neurons of the vagus nerve,” Neuroimmunomodulation, 5, 234–240 (1998).
R. P. Gaykema, S. M. Park, C. R. McKibbin, and L. E. Goehler, “Lipopolysaccharide suppresses activation of the tuberomammilary histaminergic system concomitant with behavior: a novel target of immune-sensory pathways,” Neurosci., 152, No. 1, 273–287 (2008).
L. E. Goehler, R. P. A. Gaykema, K. Hansen, J. L. Kleiner, S. F. Maier, and L. R. Watkins, “Staphylococcal enterotoxin B induces fever, brain c-Fos expression, and serum corticosterone in rats,” Am. J. Physiol. Regul. Integr. Comp. Physiol., 280, R1434–R1439 (2001).
J. J. Hagan, R. A. Leslie, S. Patel, M. L. Evans, T. A. Wattam, S. Holmes, C. D. Benham, S. G. Taylor, C. Routledge, R. P. Munton, T. E. Ashmeade, A. S. Shah, J. P. Hatcher, P. D. Hatcher, D. N. Jones, M. I. Smith, D. C. Piper, A. J. Hunter, R. A. Porter, and N. Upton, “Orexin A activates locus coeruleus cell firing and increases arousal in the rat,” Proc. Natl. Acad. Sci. USA, 96, 10911–10916 (1999).
M. Harbuz, “Neuroendocrine function and chronic inflammatory stress,” Exp. Physiol., 87, No. 5, 519–525 (2002).
J. D. Hentall, G. Zorman, S. Kansky, and H. Fields, “An estimate of minimum number of brain stem neurons required for inhibition of a flexion reflex,” J. Neurophysiol., 51, 978–985 (1984).
G. V. Hervieu, J. E. Cluderay, and D. C. Harrison, “Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord,” Neurosci., 103, 777–797 (2001).
S. J. Hopkins and N. J. Rothwell, “Cytokines and the nervous system I: expression and regulation,” Trends Neurosci., 18, 83–88 (1995).
T. L. Horvath, S. Diano, and A. N. van den Pol, “Synaptic interaction between hypocretin (orexin) and neuropeptide Y cells in the rodent and primate hypothalamus: a novel circuit implicated in metabolic and endocrine regulations,” J. Neurosci., 19, 1072–1087 (1999).
T. Ida, K. Nakahara, and T. Murakami, “Possible involvement of orexin in the stress reaction in rats,” Biochem. Biophys. Res. Commun., 270, 318–323 (2000).
M. Jaszberenyi, E. Bujdoso, and G. Telegdy, “The role of neuropeptide Y in orexin-induced hypothalamic-pituitary-adrenal activation,” J. Neuroendocrinol., 13, 438–441 (2001).
M. Jaszberenyi, E. Bujdoso, I. Pataki, and G. Telegdy, “Effects of orexins on the hypothalamic-pituitary-adrenal system,” J. Neuroendocrinol., 12, 1174–1178 (2000).
D. N. Jones, J. Gartlon, F. Parker, S. G. Taylor, C. Routledge, P. Hemmati, R. P. Munton, T. E. Ashmeade, J. P. Hatcher, A. Johns, R. A. Porter, J. J. Hagan, A. J. Hunter, and N. Upton, “Effects of centrally administered orexin-B and orexin-A: a role for orexin-1 receptors in orexin-B-induced hyperactivity,” Psychopharmacology (Berlin), 153, 210–218 (2001).
A. J. Kastin and V. Akerstrom, “Orexin A but not orexin B rapidly enters brain from blood by simple diffusion,” J. Pharmacol. Exp. Ther., 289, 219–223 (1999).
T. Kodama, S. Usui, Y. Honda, and M. Kimura, “High Fos expression during the active phase in orexin neurons of a diurnal rodent, Tamias sibiricus barberi,” Peptides, 26, No. 4, 631–638 (2005).
Y. Koyama, K. Takahashi, T. Kodama, and Y. Kayama, “State-dependent activity of neurons in the perifornical hypothalamic area during sleep and waking,” Neurosci., 119, No. 4, 1209–1219 (2003).
K. Kunii, A. Yamanaka, T. Nambu, J. Matsuzaki, K. Goto, and T. Sakurai, “Orexins/hypocretins regulate drinking behaviour,” Brain Res., 842, 256–261 (1999).
K. Kurose, Y. Ueta, Y. Yamamoto, R. Serino, Y. Ozaki, J. Saito, S. Nagata, and H. Yamashita, “Effects of restricted feeding on the activity of hypothalamic orexin (OX)-A containing neurons and OX2 receptor mRNA level in the paraventricular nucleus of rats,” Regul. Pept., 104, 145–151 (2002).
S. Lacroix and S. Rivest, “Functional circuitry in the brain of immune-challenged rats: Partial involvement of prostaglandins,” J. Comp. Neurol., 387, 307–324 (1997).
L. De Lecea, T. S. Kilduff, C. Peyron, X. Gao, P. E. Foye, P. E. Danielson, C. Fukuhara, E. L. Battenberg, V. T. Gautvik, F. S. Bartlett, W. N. Frankel, A. N. van den Pol, F. E. Bloom, K. M. Gautvik, and J. G. Sutcliffe, “The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity,” Proc. Natl. Acad. Sci. USA, 95, 322–327 (1998).
Y. Li, X. B. Gao, T. Sakurai, and A. N. van den Pol, “Hypocretin/orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system,” Neuron, 36, 1169–1181 (2002).
L. K. Malendowicz, A. Hochol, A. Ziolkowska, M. Nowak, L. Gottardo, and G. G. Nussdorfer, “Prolonged orexin administration stimulates steroid-hormone secretion, acting directly on the rat adrenal gland,” Int. J. Mol. Med., 7, 401–404 (2001).
L. K. Malendowicz, C. Tortorella, and G. G. Nussdorfer, “Orexins stimulate corticosterone secretion of rat adrenocortical cells, through the activation of the adenylate cyclase-dependent signaling cascade,” J. Steroid Biochem. Mol. Biol.,, 70, 185–188 (1999).
P. J. F. Martins, V. D’Almeida, M. Pedrazzoli, L. Lin, E. Mignot, and S. Tufik, “Increased hypocretin-1 (Orexin-A) levels in cerebrospinal fluid of rats after short-term forced activity,” Regul. Pept., 117, 155–158 (2004).
G. Mazzocchi, K. L. Malendowicz, L. Gottardo, F. Aragona, and G. G. Nussdorfer, “Orexin A stimulates cortisol secretion from human adrenocortical cells through activation of the adenylate cyclase-dependent signaling cascade,” J. Clin. Endocrinol. Metab., 86, 778–782 (2001).
S. Metanikov and V. Chorine, “Roles des reflexes conditionels dans l’immunite,” Ann. Inst. Pasteur, 40, 893–900 (1926).
T. Moriguchi, T. Sakurai, T. Nambu, M. Yanagisawa, and K. Goto, “Neurons containing orexin in the lateral hypothalamic area of the adult rat brain are activated by insulin-induced acute hypoglycemia,” Neurosci. Lett., 264, 101–104 (1999).
T. Nambu, T. Sakurai, K. Mizukami, Y. Hosoya, M. Yanagisawa, and K. Goto, “Distribution of orexin neurons in the adult rat brain,” Brain Res., 827, 243–260 (1999).
T. Nanmoku, K. Isobe, T. Sakurai, A. Yamanaka, K. Takekoshi, Y. Kawakami, K. Goto, and T. Nakai, “Effects of orexin on cultured porcine adrenal medullary and cortex cells,” Regul. Pept., 104, 125–130 (2002).
Su-Mi Park, P. A. Gaykema Ron, and L. E. Goehler, “How does immune challenge inhibit ingestion of palatable food? Evidence that systemic lipopolysaccharide treatment modulates key nodal points of feeding neurocircuitry,” Brain Behav. Immun., 22, No. 8, 1160–1172 (2008).
S. V. Perekrest, T. V. Abramova, N. S. Novikova, Yu. V. Loskutov, V. J. Rogers, and E. A. Korneva, “Changes in immunoreactivity of orexin-A-positive neurons after an intravenous lipopolysaccharide injection,” Med. Sci. Monitoring, 14, No. 7, BR127–BR133 (2008).
C. Peyron, D. K. Tighe, A. N. van den Pol, L. De Lecea, H. C. Heller, J. G. Sutcliffe, and T. S. Kilduff, “Neurons containing hypocretin (orexin) project to multiple neuronal systems,” J. Neurosci., 18, 9996–10015 (1998).
C. Phelps and L. T. Chen, “Brain response to endotoxin,” in: Cytokines and the Brain, C. Phelps and E. Korneva (eds.) (2008), pp. 435–455.
H. S. Randeva, E. Karteris, D. Grammatopoulos, and E. W. Hillhouse, “Expression of orexin-A and functional orexin type 2 receptors in the human adult adrenals: implications for adrenal function and energy homeostasis,” J. Clin. Endocrinol. Metab., 86, 4808–4813 (2001).
R. J. Rodgers, J. C. Halford, R. L. Nunes de Souza, A. L. Canto de Souza, D. C. Piper, J. R. Arch, and J. E. Blundell, “Dose-response effects of orexin-A on food intake and the behavioural satiety sequence in rats,” Regul. Pept., 96, 71–84 (2000).
S. H. Russell, M. S. Kim, C. J. Small, C. R. Abbott, D. G. Morgan, S. Taheri, K. G. Murdoch, J. F. Todd, M. A. Ghatei, and S. R. Bloom, “Central administration of orexin A suppresses basal and domperidone stimulated plasma prolactin,” J. Neuroendocrinol., 12, 1213–1218 (2000).
S. H. Russell, C. J. Small, D. Sunter, L. Morgan, C. L. Dakin, M.A. Cohen, and S. R. Bloom, “Chronic intraparaventricular nuclear administration of orexin A in male rats does not alter thyroid axis or uncoupling protein-1 in brown adipose tissue,” Regul. Pept., 104, 61–78 (2002).
E. G. Rybakina, S. N. Shanin, I. A. Kozinets, E. E. Fomicheva, and E. A. Korneva, “Cellular mechanisms of cold stress-related immunosuppression and the action of interleukin 1,” Int. J. Tiss. Reac., XIX, No. 3/4, 135–140 (1997).
F. Sakamoto, S. Yamada, and Y. Ueda, “Centrally administered orexin-A activates corticotrophin-realising factor (CRF)-containing neurons in the hypothalamic paraventricular nucleus and central amygdaloid nucleus of rats: possible involvement of central orexins on stress-activated central CRF neurons,” Regul. Pept., 118, 248–256 (2004).
T. Sakurai, A. Amemiya, M. Ishii, I. Matsuzaki, R. M. Chemelli, H. Tanaka, S. C. Williams, J. A. Richardson, G. P. Kozlowski, S. Wilson, J. R. Arch, R. E. Buckingham, A. C. Haynes, S. A. Carr, R. S. Annan, D. E. McNulty, W. S. Liu, J. A. Terrett, N. A. Elshourbagy, D. J. Bergsma, and M. Yanagisawa, “Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior,” Cell, 92, 573–585 (1998).
W. K. Samson, B. Gosnell, J. K. Chang, Z. T. Resch, and T. C. Murphy, “Cardiovascular regulatory actions of the hypocretins in brain,” Brain Res., 831, 248–253 (1999).
W. K. Samson, M. M. Taylor, M. Follwell, and A. V. Ferguson, “Orexin actions in hypothalamic paraventricular nucleus: physiological consequences and cellular correlates,” Regul. Pept., 104, 97–103 (2002).
J. F. Sheridan, N. G. Feng, R. H. Bonneau, C. M. Allen, B. S. Huneycutt, and R. Glaser, “Restrain stress differentially affects anti-viral cellular and humoral immune responses in mice,” J. Neuroimmunol., 31, No. 3, 245–255 (1991).
T. Shirasaka, M. Nakazoto, S. Matsukura, M. Takasaki, and H. Kannan, “Sympathetic and cardiovascular actions of orexins in conscious rats,” Am. J. Physiol. Regul. Integr. Comp. Physiol., 277, 1780–1785 (1999).
U. Steidl, S. Bork, S. Schaub, et al., “Primary human CD34+ hematopoietic stem and progenitor cells express functionally active receptors of neuromediators,” Blood, 104, No. 1, 81–88 (2004).
T. L. Steininger, T. S. Kilduff, M. Behan, R. M. Benca, and C. F. Landry, “Comparison of hypocretin/orexin and melanin-concentrating hormone neurons and axonal projections in the embryonic and postnatal rat brain,” J. Chem. Neuroanat., 27, No. 3, 165–181 (2004).
N. Takahashi, T. Okumura, H. Yamada, and Y. Kohgo, “Stimulation of gastric acid secretion by centrally administered orexin-A in conscious rats,” Biochem. Biophys. Res. Commun., 254, 623–627 (1999).
R. Tamura, T. Kondoh, T. Ono, H. Nishijo, and K. Torii, “Effects of repeated cold stress on activity of hypothalamic neurons in rats during performance of operant licking task,” J. Neurophysiol., 84, No. 6, 2844–2858 (2000).
P. Trivedi, H. Yu, D. J. MacNeil, L. H. Van der Ploeg, and X. M. Guan, “Distribution of orexin receptor mRNA in the rat brain,” FEBS Lett., 438, 71–75 (1998).
A. V. Turnbull and C. L. Rivier, “Regulation of the hypothalamicpituitary-adrenal axis by cytokines: Actions and mechanisms of action,” Physiol. Rev., 79, No. 1, 1–71 (1999).
A. N. van den Pol, “Hypothalamic hypocretin (orexin): robust innervation of the spinal cord,” J. Neurosci., 19, 3171–3182 (1999).
A. N. van den Pol, X. B. Gao, K. Obrietan, T. S. Kilduff, and A. B. Belousov, “Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin,” J. Neurosci., 18, 7962–7971 (1998).
D. M. Webel, B. N. Finck, H. D. Baker, and R. W. Johnson, “Time course of increased plasma cytokines, cortisol, and urea nitrogen in pigs following intraperitoneal injection of lipopolysaccharide,” J. Anim. Sci., 75, 1514–1520 (1997).
S. Zhang, D. Blache, P. E. Vercoe, C. L. Adam, M. A. Blackberry, P. A. Findlay, K. A. Eidne, and G. B. Martin, “Expression of orexin receptors in the brain and peripheral tissues of the male sheep,” Regul. Pept., 124, 81–87 (2005).
Y.-H. Zhang, J. K. Jan Lu, J. K. Elmquist, and C. B. Saper, “Lipopolysaccharide activates specific populations of hypothalamic and brainstem neurons that project to the spinal cord,” J. Neurosci., 20, No. 17, 6578–6586 (2000).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 95, No. 12, pp. 1309–1323, December, 2009.
Rights and permissions
About this article
Cite this article
Novikova, N.S., Perekrest, S.V., Shainidze, K.Z. et al. Hypothalamic Orexin-Containing Neurons in the Hypothalamus on Exposure to Antigenic and Non-Antigenic Stimuli. Neurosci Behav Physi 41, 188–197 (2011). https://doi.org/10.1007/s11055-011-9399-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-011-9399-4