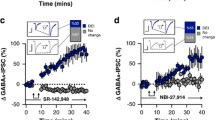
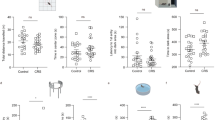
Corticoliberin (corticotrophin-releasing factor, CRF, CRH) is an active regulator of endocrine, autonomic, and immune functions in stress, as well as a mediator of anxiety, determining the behavioral stress response. The present report describes studies of its action on neuron activity evoked by microstimulation of olfactory cortex slices. Behavioral testing in a T maze was used to select individuals with a passive behavioral strategy from a population of Wistar rats, and the animals were subjected to water immersion. Olfactory cortex slices were prepared 10 days later and evoked focal potentials were recorded on perfusion with medium containing corticoliberin (0.1 μM). Among active rats, 60% of slices retained high excitability after stress, and corticoliberin produced only insignificant reductions in the amplitudes of excitatory potentials in these slices, simultaneously increasing the amplitudes of inhibitory potentials. Low excitability was found in 40% of slices from active stressed rats, and corticoliberin had a significant inhibitory effect in these slices. Addition of corticoliberin to the incubation medium used for slices from passive rats with initially low excitability led to complete blockade of synaptic transmission. These data support the involvement of corticoliberin in the development of depression.
Similar content being viewed by others
References
V. I. Mironova, E. A. Rybnikova, V. V. Rakitskaya, and V. G. Shalyapina, “Corticoliberin contents in the hypothalamus of rats with different strategies of adaptive behavior in post-stress depression,” Ros. Fiziol. Zh. im. I. M. Sechenova, 90¸ No. 9, 1161–1163 (2004).
M. I. Mityushov, N. A. Emel’yanov, A. A. Mokrushin, I. A. Voiner, and T. R. Bagaeva, Living Brain Slices [in Russian], Nauka, Leningrad (1986).
O. G. Semenova, V. V. Rakitskaya, and V. G. Shalyapina, “Blockade of corticosterone receptors prevents the development of post-stress psychopathology in rats with an active strategy of adaptive behavior,” Ros. Fiziol. Zh. im. I. M. Sechenova, 92, No. 11, 1345–1349 (2006).
O. G. Semenova, M. G. Semenova, V. V. Rakitskaya, and V. G. Shalyapina, “Psychomotor reactivity to corticoliberin in rats with active and passive strategies of adaptive behavior in a water immersion model of depression,” Ros. Fiziol. Zh. im. I. M. Sechenova, 92, No. 8, 1016–1021 (2006).
V. G. Shalyapina, “Corticoliberin in the regulation of adaptive behavior and the pathogenesis of post-stress psychopathology,” in: Basic Neuroendocrinology [in Russian], Élbi, St. Petersburg (2005), pp. 84–146.
V. G. Shalyapina, E. A. Vershinina, V. V. Rakitskaya, L. Yu. Ryzhova, M. G. Semenova, and O. G. Semenova, “Changes in adaptive behavior in active and passive Wistar rats in a water immersion model of depression,” Zh. Vyssh. Nerv. Deyat., 56, No. 4, 543–547 (2006).
V. G. Shalyapina, E. A. Vershinina, V. V. Rakitskaya, L. Yu. Ryzhova, M. G. Semenova, and O. G. Semenova, “Changes in adaptive behavior in active and passive Wistar rats in a water immersion model of depression,” Zh. Vyssh. Nerv. Deyat., 56, No. 4, 543–547 (2006).
V. G. Shalyapina, A. A. Mokrushina, and N. N. Nesterov, “Corticoliberin protects neurons in living olfactory cortex slices from the negative effects of dysfunctins,” Ros. Fiziol. Zh. im. I. M. Sechenova, 88, No. 3, 332–338 (2002).
V. G. Shalyapina,V. V. Rakitskaya, and E. I. Petrova, “Corticotropinreleasing hormone in changes in the behavioral sequelae of unavoidable stress in active and passive rats,” Zh. Vyssh. Nerv. Deyat., 55, No. 2, 241–244 (2005).
V. G. Shalyapina, V. V. Rakitskaya, M. G. Semenova, and O. G. Semenova, “Hormonal function of the hypophyseal-adrenocortical system in the pathogenetic heterogeneity of post-stress depression,” Ros. Fiziol. Zh. im. I. M. Sechenova, 92, No. 4, 480–487 (2006).
M. W. Croighead, H. Bouten, R. M. Middlehurst, and S. M. Allan, “Influence of corticotrophin-releasing factor on neuronal cell death in vitro and in vivo,” Brain Res., 881, No. 2, 139–143 (2000).
E. R. De Kloet, “Hormones and stressed brain,” Ann. N.Y. Acad. Sci., 1018, 1–15 (2004).
P. W. Gold, M. L. Wong, G. P. Chroussos, and J. Liciano, “Stress system abnormalities in melancholic and atypical depression: molecular pathophysiological and therapeutic implication,” Med. Psychiatry, 1, 257–264 (1996).
W. H. Hoffman and L. B. Haberly, “Bursting induces persistent All-or-None EPSPs by an NMDA-dependent process in piriform cortex,” J. Neurosci., 9, No. 1, 206–215 (1989).
M. Joels, J. Krugers, and J. M. Verkuyl, “Modulation of glutamatergic and GABA-ergic neurotransmission by corticosteroid hormones and stress,” in Handbook of Stress and Brain, T. Steekler, N. H. Kabia, and J. M. Reul (eds.) (2005), Vol. 15, pp. 525–544.
M. W. Jung, J. Larson, and G. Lynch, “Role of NMDA and non-NMDA receptors in synaptic transmission in rat piriform cortex,” Exptl. Brain Res., 82, No. 5, 451–455 (1990).
J. W. Kaskow, D. Daker, and N. C. Geracioti, “Corticotropin-releasing hormone in depression and post-traumatic stress disorder,” Peptides, 22, 845–851 (2001).
C. B. Nemeroff, “The corticotropin-releasing factor (CRF) hypothesis of depression: finding and new directions,” Mol. Psychiatry, 1, 336–342 (1996).
F. C. Raadsheer,W. S. Hoogendijk, and F. C. Stam, “Increased number of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients,” Neuroendocrinol., 60, 433–435 (1994).
R. M. Sapolsky, L. Krey, and B. S. McEwen, “The neurobiology of stress and aging; the glucocorticoids cascade hypothesis,” Endocrinol. Rev., 7, 284–301 (1986).
K. Takagi, Y. Kasuya, and K. Watanabe, “Studies on the drug for peptide ulcer, a reliable method for production stress ulcer,” Chem. Pharmacol. Bull., 12, 465–473 (1964).
G. F. Tseng and L. B. Haberly, “Characterization of synaptically mediated fast and slow inhibitory processes in piriform cortex in an in vitro slice preparation,” J. Neurophysiol., 59, No. 5, 1352–1376 (1988).
D. T. Usually, F. L. Chen, and L. K. Takahashi, “Rapid stress induced elevation in corticotrophin-releasing hormone mRNA in rat central amygdaloidal nucleus and hypothalamic paraventricular nucleus: an in situ hybridization analysis,” Brain Res., 788, 305–310 (1988).
R. Yuhuda, “Advances in understanding neuroendocrine alteration in PTSD and their therapeutic implication,” Ann. N.Y. Acad. Sci., 1071, 137–166 (2006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Rossiiskii Fiziologicheskii Zhurnal imeni I. M. Sechenova, Vol. 94, No. 8, pp. 952–961, August, 2008.
Rights and permissions
About this article
Cite this article
Shalyapina, V.G., Mokrushin, A.A., Khama-Murad, A.K. et al. Effects of Corticoliberin on Synaptic Transmission in Rat Olfactory Cortex Slices in a Water Immersion Model of Depression. Neurosci Behav Physi 39, 701–707 (2009). https://doi.org/10.1007/s11055-009-9176-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-009-9176-9