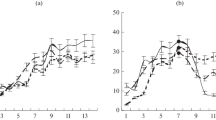
Male C57BL/6J, BALB/c, and DBA/2J mice showed differences in their abilities to perform two cognitive tests. C57BL/6J mice had good learning ability and memory trace retention (at 10 days) in a simplified Morris maze, while BALB/c mice had low levels of memory trace retention and DBA/2J mice had low learning ability in this test. I.p. administration of the nootropic agent Noopept (GVS-111, N-phenylacetyl-L-prolylglycine ethyl ester) at a dose of 0.5 mg/kg 15 min before the start of the test induced significant improvements in long-term memory in this test in BALB/c mice but no further improvement in C57BL/6J mice, and had no effect in DBA/2J mice. On testing the ability to extrapolate the direction of movement of a stimulus, administration of Noopept increased the proportion of correct responses in C57BL/6J and BALB/c mice, but had no effect in DBA/2J mice.
Similar content being viewed by others
References
A. P. Bel'nik, R. U. Ostrovskaya, and I. I. Poletaeva, “The behavior of mice of different strains — modification by Noopept,” Zh. Vyssh. Nerv. Deyat., 57, No. 6, (2007).
T. A. Voronina and R. U. Ostrovskaya, Methods for the Study of the Nootropic Activity of Pharmacological Substances. Handbook for Experimental (Preclinical) Studies of Novel Pharmacological Agents [in Russian], R. U. Khabriev (ed.), Moscow (2005), pp. 308–320.
L. V. Krushinskii, A. P. Dyban, I. I. Poletaeva, L. G. Romanova, N. V. Papova, and V. S. Baranov, “Approaches to the physiological-genetic study of the ability of mice to extrapolate,” Zh. Vyssh. Nerv. Deyat., 28, No. 5, 903–912 (1978).
G. F. Lakin, Biometrics [in Russian], Vysshaya Shkola, Moscow (1990).
I. G. Lil'p, F. Z. Blizikoeva, I. I. Poletaeva, and V. I. Ivanov, “Interstrain differences in learning ability in 101/H and CBA mice in a water maze (modified Morris test),” Byull. Éksperim. Biol. Med., 124, No. 12, 666–668 (1997).
R. U. Ostrovskaya, T. A. Gudasheva, T. A. Voronina, and S. B. Seredinin, “An original nootropic and neuroprotective agent,” Éksperim. Klin. Farmakol., No. 5, 66–72 (2002).
R. U. Ostrovskaya, T. Kh. Mirzoev, F. A. Firova, S. S. Trofimov, T. A. Gudasheva, T. N. Grechenko, E. F. Gutyrchik, and E. B. Barkova, “Behavioral and electrophysiological analysis of the cholinepositive action of the nootropic acyl-proline dipeptide GVS-111,” Éksperim. Klin. Farmakol., 64, No. 2, 11–14 (2001).
O. V. Perepelkina, N. V. Markina, and I. I. Poletaeva, “Ability to extrapolate movement direction in mice bred for large and small brain weight: effects of being kept in a ‘rich’ environment,” Zh. Vyssh. Nerv. Deyat., 56, No. 2, 282–286 (2006).
N. V. Popova and I. I. Poletaeva, “Ability to solve an extrapolation task in mice bred for large and small brain weight,” Zh. Vyssh. Nerv. Deyat., 33, No. 2, 370–372 (1983).
S. B. Seredenin, Lectures in Pharmacogenetics [in Russian], Medical Information Agency, Moscow (2004).
S. B. Seredenin, B. A. Badyshtov, G. G. Neznamov, A. L. Makhnysheva, I. V. Kolotilinskaya, and S. N. Nadorov, “Prediction of individual reactions to emotional stress and benzodiazepine tranquilizers,” Éksperim. Klin. Farmakol., 64, No. 1, 3–12 (2001).
M. Ammassari-Teule and A. Caprioli, “Spatial learning and memory, maze running strategies and cholinergic mechanisms in two inbred mouse strains,” Behav. Brain Res., 17, No. 1, 9–16 (1985).
M. Arns, M. Sauvage, and T. Steckler, “Excitotoxic hippocampal lesions disrupt allocentric spatial learning in mice: effects of strain and task demands,” Behav. Brain Res., 106, No. 1–2, 151–164 (1999).
N. De Bruin, M. Mahieu, T. Patel, R. Willems, A. Lesage, and A. Megens, “Performance of F2 B6x129 hybrid mice in the Morris water maze, latent inhibition and prepulse inhibition paradigms: Comparison with C57B1/6J and 129/sv inbred mice,” Behav. Brain Res., 172, No. 1, 122–134 (2006).
D. E. Fordyce, V. J. Clark, R. Paylor, and J. M. Wehner, “Enhancement of hippocampally-mediated learning and protein kinase C activity by oxiracetam in learning-impaired DBA/2 mice,” Brain Res., 672, No. 1, 170–176 (1995).
T. A. Gudasheva, T. A. Voronina, R. U. Ostrovskaya, G. G. Rozantsev, N. I. Vasilevich, S. S. Trofimov, E. V. Kravchenko, A. P. Skoldinov, and S. B. Seredenin, “Synthesis and antiamnesic activity of a series of N-acylprolyl-containing dipeptides,” Eur. J. Med. Chem., 31, No. 2, 151–157 (1996).
D. K. Ingram and T. P. Corfman, “An overview of neurobiological comparisons in mouse strains,” Neurosci. Biobehav. Rev., 4, 421–435 (1980).
S. Matsuyama, U. Namgung, and A. Routtenberg, “Long-term potentiation persistence greater in C57BL/6J/6 than DBA/2J mice: predicted on basis of protein kinase C levels and learning performance,” Brain Res., 763, 127–130 (1997).
R. G. M. Morris, “Development of water-maze procedure for studying spatial learning in the rat,” J. Neurosci. Meth., 11, 47–60 (1984).
L. Nadel, “The role of the hippocampus in declarative memory: a comment on Zola-Morgan, Squire, and Ramus,” Hippocampus, 5, 232–239 (1995).
E. Passino, S. Middei, L. Restivo, V. Bertaina-Anglade, and M. Ammassari-Teule, “Genetic approach to variability of memory systems: analysis of place vs. response learning and fos-related expression in hippocampal and striatal areas of C57BL/6 and DBA/2 mice,” Hippocampus, 12, No. 1, 63–75 (2002).
I. I. Poletaeva, N. V. Popova, and L. G. Romanova, “Genetic aspects of animal reasoning,” Behav. Genet., 23, 467–475 (1993).
I. I. Poletaeva, E. I. Sarychev, L. G. Alfeeva, and M. M. Kozlovskaya, “Logic task solution and noothropic drug effect,” in: Signal Molecules and Behavior, W. Winslow, O. Vinogradova, and D. Sakharov (eds.), Manchester University Press, Manchester (1991), pp. 278–285.
S. S. R. Rose, “'smart drugs'-do they work?” Nature Rev. Neurosci., 3, 975–979 (2002).
H. Schwegler, W. E. Crusio, H.-P. Lipp, and B. Heimrich, “Water-maze learning in the mouse correlates with variation in hippocampal morphology,” Behav. Genet., 18, 153–166 (1988).
L. A. Schimanski and P. V. Nguyen, “Multidisciplinary approaches for investigating the mechanisms of hippocampus-dependent memory: a focus on inbred mouse strains,” Behav. Brain Res., 172, No. 1, 122–134 (2006).
S. B. Seredenin, T. A. Voronina, T. A. Gudasheva, R. U. Ostrovskaya, G. G. Rozantsev, A. P. Skoldinov, S. S. Trofimov, J. A. Halikas, and G. L. Garibova, “N-Acylprolyl-dipeptides having antiamnestic, antihypoxic and anorexigenic effects,” US Patent No. 5,439,930 (1995).
M. Upchurch and J. M. Wehner, “Inheritance of spatial learning ability in inbred mice: a classical genetic analysis,” Behav. Neurosci., 103, No. 6, 1251–1258 (1989).
D. Van Dam, D. Abramowski, M. Staufenbiel, and P. P. De Deyn, “Symptomatic effect of donepezil, rivastigmine, galantamine and memantine on cognitive deficits in the APP23_model,” Psychopharmacology (Berlin), 180, No. 1, 177–190 (2005).
M. Yoshida, G. Goto, and S. Watanabe, “Task-dependent strain difference of spatial learning in C57BL/6J and BALB/c mice,” Physiol. Behav., 73, No. 1–2, 37–42 (2001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Vysshei Nervnoi Deyatel'nosti imeni I. P. Pavlova, Vol. 57, No. 6, pp. 721–728, November–December, 2007.
Rights and permissions
About this article
Cite this article
Bel’nik, A.P., Ostrovskaya, R.U. & Poletaeva, I.I. Genotype-dependent characteristics of behavior in mice in cognitive tests. The effects of Noopept. Neurosci Behav Physi 39, 81–86 (2009). https://doi.org/10.1007/s11055-008-9095-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-008-9095-1