Abstract
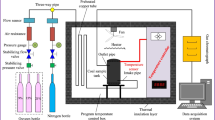
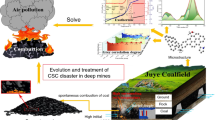
Dynamic hazard and coal spontaneous combustion are major potential safety hazards in coal mines with enriched gas and spontaneously inflammable property. However, the initial oxidizability of coal samples after dynamic hazard has yet to be understood. A simplified device for dynamic hazard simulation of coal-and-gas outburst was established. The physical and chemical changes of coal surface, and their effects on initial oxidation were analyzed. Firstly, loose coal was pressurized by gas step-by-step, and the CO content was enhanced with increase in gas pressure. Meanwhile, the specific surface area provides a hotbed for low-temperature oxidation of radicals and functional groups of coal. Finally, the mechanism of mechanical force on the increase of radical and active groups with the homolysis of covalent bond was discussed. The initial oxidizability of coal after dynamic damage was characterized by oxygen absorption. The physicochemical characteristics suitable for coal oxidation, were formed under the mechano–chemical effect. The findings presented in here add to our understanding of the influence of dynamic hazard on coal oxidation.











Similar content being viewed by others
References
Baláž, P., & Dutková, E. (2009). Fine milling in applied mechanochemistry. Minerals Engineering, 22(7–8), 681–694.
Baláž, P. (2008). Mechanochemistry in nanoscience and minerals. Springer.
Beyer, M. K., & Clausen-Schaumann, H. (2005). Mechanochemistry: The mechanical activation of covalent bonds. Chemical Reviews, 105(8), 2921–2948.
Bolm, C., & Hernández, J. G. (2019). Mechanochemistry of gaseous reactants. Angewandte Chemie (international Ed. in English), 58(11), 3285–3299.
Brunauer, S., Emmett, P. H., & Teller, E. (1938). Adsorption of gases in multimolecular layers. Journal of American Chemical Society, 60(2), 309–319.
Cao, D., Li, X., & Zhang, S. (2007). Influence of tectonic stress on coalification: Stress degradation mechanism and stress polycondensation mechanism. Science in China Series D: Earth Sciences, 50(1), 43–54.
Cao, Y., Davis, A., Liu, R., Liu, X., & Zhang, Y. (2003). The influence of tectonic deformation on some geochemical properties of coals—a possible indicator of outburst potential. International Journal of Coal Geology, 53, 69–79.
Cao, Y., Mitchell, G. D., Davis, A., & Wang, D. (2000). Deformation metamorphism of bituminous and anthracite coals from China. International Journal of Coal Geology, 43, 227–242.
Carr, R. M., Kumagai, H., Peake, B. M., Robinson, B. H., Clemens, A. H., & Matheson, T. W. (1995). Formation of free radicals during drying and oxidation of a lignite and a bituminous coal. Fuel, 74(3), 389–394.
Chu, T., Li, P., & Chen, Y. (2019). Risk assessment of gas control and spontaneous combustion of coal under gas drainage of an upper tunnel. International Journal of Mining Science and Technology, 29(3), 491–498.
Dack, S. W., Hobday, M. D., Smith, T. D., & Pilbrow, J. R. (1985). E.p.r study of organic free radicals in Victorian brown coal. Fuel, 64(2), 219–221.
Davis, D. A., Hamilton, A., Yang, J., Cremar, L. D., Van Gough, D., Potisek, S. L., et al. (2009). Force-induced activation of covalent bonds in mechanoresponsive polymeric materials. Nature, 459(7243), 68–72.
Friščić, T., Mottillo, C., & Titi, H. M. (2020). Mechanochemistry for synthesis. Angewandte Chemie (international Ed. in English), 59(3), 1018–1029.
Ge, T., Zhang, M., & Cai, C. (2014). XPS analysis of surface texture of coking coal in Fenxi County. Asian Journal of Chemistry, 26(6), 1741–1744.
Grzybek, T., Pietrzak, R., & Wachowska, H. (2004). The comparison of oxygen and sulfur species formed by coal oxidation with O-2/Na2CO3 or peroxyacetic acid solution. XPS Studies. Energy & Fuels, 18(3), 804–809.
Hou, Q., Han, Y., Wang, J., Dong, Y., & Pan, J. (2017). The impacts of stress on the chemical structure of coals: A mini-review based on the recent development of mechanochemistry. Science Bulletin, 62(13), 965–970.
Hu, X. C., Yang, S. Q., Liu, W. V., Zhou, X. H., Sun, J. W., & Yu, H. (2017). A methane emission control strategy in the initial mining range at a spontaneous combustion-prone longwall face: A case study in coal 15, Shigang Mine, China. Journal of Natural Gas Science and Engineering, 38, 504–515.
Jiang, X. M., Zheng, C. G., Yan, C., Liu, D. C., Qiu, J. R., & Li, J. B. (2002). Physical structure and combustion properties of super fine pulverized coal particle. Fuel, 81(6), 793–797.
Kaupp, G. (2009). Mechanochemistry: The varied applications of mechanical bond-breaking. CrystEngComm, 11(3), 388–403.
Kitamura, M., Mukoyoshi, H., Fulton, P. M., & Hirose, T. (2012). Coal maturation by frictional heat during rapid fault slip. Geophysical Research Letters, 39(16), L16302.
Kong, B., Wang, E., Lu, W., & Li, Z. (2019). Application of electromagnetic radiation detection in high-temperature anomalous areas experiencing coalfield fires. Energy, 189, 116144.
Li, J., Li, Z., Yang, Y., Wang, C., & Sun, L. (2018). Experimental study on the effect of mechanochemistry on coal spontaneous combustion. Powder Technology, 339, 102–110.
Li, X., Ju, Y., Hou, Q., & Lin, H. (2012). Spectra response from macromolecular structure evolution of tectonically deformed coal of different deformation mechanisms. Science China Earth Sciences, 55(8), 1269–1279.
Ma, L., Wang, D., Wang, Y., Xin, H., Dou, G., & Xu, C. (2016). Experimental investigation on a sustained release type of inhibitor for retarding the spontaneous combustion of coal. Energy & Fuels, 30(11), 8904–8914.
Mathews, J. P., & Chaffee, A. L. (2012). The molecular representations of coal—A review. Fuel, 96, 1–14.
Qi, X., Chen, L., Xin, H., Ji, Y., Bai, C., Song, R., et al. (2018). Reaction mechanism and thermodynamic properties of aliphatic hydrocarbon groups during coal self-heating. Energy & Fuels, 32(10), 10469–10477.
Qin, B. T., Li, L., Ma, D., Lu, Y., Zhong, X. X., & Jia, Y. W. (2016). Control technology for the avoidance of the simultaneous occurrence of a methane explosion and spontaneous coal combustion in a coal mine: A case study. Process Safety and Environmental Protection, 103, 203–211.
Seehra, M. S., Ghosh, B., Zondlo, J. W., & Mintz, E. A. (1988). Relationship of coal extraction with free radicals and coal macerals. Fuel Processing Technology, 18(n), 279–286.
Song, Y., Jiang, B., & Han, Y. (2018). Macromolecular response to tectonic deformation in low-rank tectonically deformed coals (TDCs). Fuel, 219, 279–287.
Tadyszak, K., Augustyniak-Jabłokow, M. A., Więckowski, A. B., Najder-Kozdrowska, L., Strzelczyk, R., & Andrzejewski, B. (2015). Origin of electron paramagnetic resonance signal in anthracite. Carbon, 94, 53–59.
Tang, Y., & Wang, H. (2018). Development of a novel bentonite-acrylamide superabsorbent hydrogel for extinguishing gangue fire hazard. Powder Technology, 323, 486–494.
Wang, C., Yang, S., Li, X., Jiang, C., & Li, M. (2018). Study on the failure characteristics of concrete specimen under confining pressure. Arabian Journal for Science and Engineering, 44(5), 4119–4129.
Wang, J., Guo, G.-J., Han, Y., Hou, Q., Geng, M., & Zhang, Z. (2019). Mechanolysis mechanisms of the fused aromatic rings of anthracite coal under shear stress. Fuel, 253, 1247–1255.
Xi, X., Jiang, S., Zhang, W., Wang, K., Shao, H., & Wu, Z. (2019). An experimental study on the effect of ionic liquids on the structure and wetting characteristics of coal. Fuel, 244, 176–183.
Xia, W., Li, Y., & Niu, C. (2018). Effects of high-temperature oxygen-deficient oxidation on the surface properties of sub-bituminous coal. Energy Sources, Part a: Recovery, Utilization, and Environmental Effects, 41(9), 1110–1115.
Xu, C., Zhou, G., & Qiu, H. (2017). Analysis of the microscopic mechanism of coal wettability evolution in different metamorphic states based on NMR and XPS experiments. RSC Advances, 7(76), 47954–47965.
Xu, R., Li, H., Guo, C., & Hou, Q. (2014). The mechanisms of gas generation during coal deformation: Preliminary observations. Fuel, 117, 326–330.
Yan, F., Xu, J., Peng, S., Zou, Q., Li, Q., Long, K., & Zhao, Z. (2020). Effect of capacitance on physicochemical evolution characteristics of bituminous coal treated by high-voltage electric pulses. Powder Technology, 367, 47–55.
Yang, Y., Pan, J., Wang, K., & Hou, Q. (2020). Macromolecular structural response of Wender coal under tensile stress via molecular dynamics. Fuel, 265, 116938.
Yu, M., Xie, J., & Jia, H. (2017). Release laws of CO produced by coal structure destruction under mechanical force and modification method of the index for predicting coal spontaneous combustion. Journal of China University of Mining and Technology, 46(4), 762–768.
Zhang, L., Li, Z., He, W., Li, J., Qi, X., Zhu, J., et al. (2018). Study on the change of organic sulfur forms in coal during low-temperature oxidation process. Fuel, 222, 350–361.
Zhou, B., Yang, S., Wang, C., Hu, X., Song, W., Cai, J., et al. (2020). The characterization of free radical reaction in coal low-temperature oxidation with different oxygen concentration. Fuel, 262, 116524.
Zhou, G., Xu, C., Cheng, W., Zhang, Q., & Nie, W. (2015). Effects of oxygen element and oxygen-containing functional groups on surface wettability of coal dust with various metamorphic degrees based on XPS experiment. Journal of Analytical Methods in Chemistry, 2015, 467242.
Zhurkov, S. K., & Korsukov, V. E. (1974). Atomic mechanism of fracture of solid polymers. Journal of Polymer Science: Polymer Physics Edition, 12, 385–398.
Acknowledgments
This research was supported by the National Key R&D Program of China (2018YFC0807900) and “Double First Rate” Independent Innovation Project of CUMT (2018ZZCX05).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, J., Yang, S., Zheng, W. et al. Risk Assessment of Oxidizability of Coal after Dynamic Hazard and Its Effect on Functional Groups and Radicals. Nat Resour Res 30, 4533–4545 (2021). https://doi.org/10.1007/s11053-021-09941-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11053-021-09941-2