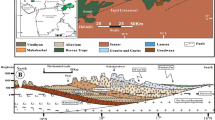
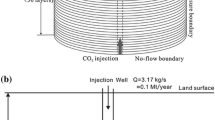
Abstract
CO2 geological storage (CGS) is a crucial strategy for meeting CO2 emission reduction targets. However, the effect of microbial action on CGS in deep saline aquifer is unclear. In this paper, numerical simulations based on previous experimental data were conducted to investigate CO2 solubility-trapping and mineral-trapping by microbial-mediated process in CGS demonstration base in Ordos, north of China. The simulation results reveal that the presence of a microbial community has a positive influence on CGS, mainly in terms of the amount of CO2 that can be injected and the manner by which it is stored. With the effect of microbial action, the amount of injected CO2 was increased by 23.33% and the CO2 mineral sequestration increased from 1.12 to 2.05%. In addition, microbial action plays different roles at different stages of CGS. In the initial stage of CO2 injection, the microbial action promotes dissolution of calcite, oligoclase and clay minerals such as chlorite and kaolinite, providing a favorable condition (more K+, Ca2+, Na+, Mg2+, Fe2+ and HCO3−) for CO2 mineral-trapping. In the following stage of CGS, microbial action accelerates the precipitation of carbon-fixing minerals such as calcite and siderite, enhancing the CO2 storage security in deep saline aquifer. As such, secondary minerals such as ferroan dolomite and dawsonite were observed in advance in microbially mediated CO2–saline–sandstone interactions, further indicating that microbes play a positive role in CO2 mineral-trapping. Consequently, the microbial action can promote mineral capture of CO2 and increase the CO2 storage security in deep saline aquifer. These conclusions can provide a reference and basis for the suitability assessment of CGS in such deep saline aquifer like Erdos, China.










Similar content being viewed by others
References
Abidoye, L. K., Khudaida, K. J., & Das, D. B. (2015). Geological carbon sequestration in the context of two-phase flow in porous media: A review. Critical Reviews in Environmental Science and Technology, 45(11), 1105–1147.
Ahmed, W., & Rodriguez, J. (2017). Generalised parameter estimation and calibration for biokinetic models using correlation and single variable optimisations: Application to sulfate reduction modelling in anaerobic digestion. Water Research, 122, 407.
Bachu, S. (2015). Review of CO2 storage efficiency in deep saline aquifers. International Journal of Greenhouse Gas Control, 40, 188–202.
Bachu, S., & Adams, J. J. (2003). Sequestration of CO in geological media in response to climate change: Capacity of deep saline aquifers to sequester CO in solution. Energy Conversion and Management, 44(20), 3151–3175.
Batstone, D. J., Keller, J., Angelidaki, I., Kalyuzhnyi, S. V., Pavlostathis, S. G., Rozzi, A., et al. (2002). The IWA anaerobic digestion model No 1 (ADM1). Water Science and Technology., 6, 10.
Bhattacharya, S., Bachani, P., Jain, D., Patidar, S. K., & Mishra, S. (2016). Extraction of potassium from K-feldspar through potassium solubilization in the halophilic Acinetobacter soli (MTCC 5918) isolated from the experimental salt farm. International Journal of Mineral Processing, 152, 53–57. https://doi.org/10.1016/j.minpro.2016.05.003.
Birkholzer, J. T., Oldenburg, C. M., & Zhou, Q. (2015). CO2 migration and pressure evolution in deep saline aquifers. International Journal of Greenhouse Gas Control, 40, 203–220.
Blake, R. E., & Walter, L. M. (1999). Kinetics of feldspar and quartz dissolution at 70–80 °C and near-neutral pH: effects of organic acids and NaCl. Geochimica et Cosmochimica Acta, 63(13–14), 2043–2059.
Boot-Handford, M. E., Abanades, J. C., Anthony, E. J., Blunt, M. J., Brandani, S., Dowell, N. M., et al. (2013). Carbon capture and storage update. Energy and Environmental Science, 7(1), 130–189.
Chen, Y., Mi, T., Liu, Y., Li, S., & Zhen, Y. (2020). Microbial community composition and function in sediments from the Pearl River mouth Basin. Journal of Ocean University of China, 19(4), 941–953.
Curtis, G. P. (2003). Comparison of approaches for simulating reactive solute transport involving organic degradation reactions by multiple terminal electron acceptors. Computers and Geosciences, 29(3), 319–329.
Das, S., Liu, C. C., Jean, J. S., Lee, C. C., & Yang, H. J. (2016). Effects of microbially induced transformations and shift in bacterial community on arsenic mobility in arsenic-rich deep aquifer sediments. Journal of Hazardous Materials, 310, 11–19.
Deng, L., Wang, F., Dong, H., Wang, H., Zhao, L., Huang, L., et al. (2016). Biological reduction of structural Fe(III) in smectites by a marine bacterium at 0.1 and 20 MPa. Chemical Geology, 438, 1–10.
Ebeling, J. M., Timmons, M. B., & Bisogni, J. J. (2006). Engineering analysis of the stoichiometry of photoautotrophic, autotrophic, and heterotrophic removal of ammonia-nitrogen in aquaculture systems. Aquaculture, 257(1–4), 346–358.
Fredrickson, J. K., & Gorby, Y. A. (1996). Environmental processes mediated by iron-reducing bacteria. Current Opinion in Biotechnology, 7(3), 287–294.
Freedman, A. J. E., Tan, B. F., & Thompson, J. R. (2017). Microbial potential for carbon and nutrient cycling in a geogenic supercritical carbon dioxide reservoir. Environmental Microbiology, 19(6), 28.
Gadikota, G., Matter, J., Kelemen, P. B., Brady, P. V., & Park, A.-H. A. (2020). Elucidating the differences in the carbon mineralization behaviors of calcium and magnesium bearing alumino-silicates and magnesium silicates for CO2 storage. Fuel, 277, 5.
Hellingwerf, K. J., Lolkema, J. S., Otto, R., Neijssel, O. M., Stouthamer, A. H., Harder, W., et al. (1982). Energetics of microbial growth: An analysis of the relationship between growth and its mechanistic basis by mosaic non-equilibrium thermodynamics. FEMS Microbiology Letters, 1, 264–271.
Huntingford, C., & Mercado, L. M. (2016). High chance that current atmospheric greenhouse concentrations commit to warmings greater than 1.5 °C over land. Scientific Reports, 6, 30294.
Jing, Z., Wei, L., Zhang, F., Cong, L., Du, J., Zhu, R., et al. (2014). Evaluation of CO2 solubility-trapping and mineral-trapping in microbial-mediated CO2 –brine–sandstone interaction. Marine Pollution Bulletin, 85(1), 78–85.
Kalyuzhnyi, S. V., & Fedorovich, V. V. (1998). Mathematical modelling of competition between sulphate reduction and methanogenesis in anaerobic reactors. Bioresource Technology, 65(3), 227–242.
Ko, M. S., Cho, K., Jeong, D., & Lee, S. (2016). Identification of the microbes mediating Fe reduction in a deep saline aquifer and their influence during managed aquifer recharge. Ence of The Total Environment, 545, 486–492.
Leigh Mascarelli, A. (2009). Geomicrobiology: Low life. Nature, 459(7248), 770–773.
Leung, D. Y. C., Caramanna, G., & Maroto-Valer, M. M. (2014). An overview of current status of carbon dioxide capture and storage technologies. Renewable and Sustainable Energy Reviews, 39(39), 426–443.
Li, C., Zhong, S., Zhang, F., Wang, Z., Jiang, F., & Wan, Y. (2017). Response of microbial communities to supercritical CO2 and biogeochemical influences on microbially mediated CO2-saline-sandstone interactions. Chemical Geology, 473, 1–9. https://doi.org/10.1016/j.chemgeo.2017.09.026.
Liu, L., Lee, D. J., Wang, A., Ren, N., Su, A., & Lai, J. Y. (2016). Isolation of Fe(III)-reducing bacterium, Citrobacter sp. LAR-1, for startup of microbial fuel cell. International Journal of Hydrogen Energy, 41(7), 4498–4503.
Liu, F., Lu, P., Griffith, C., Hedges, S. W., Soong, Y., Hellevang, H., et al. (2012). CO2–brine–caprock interaction: Reactivity experiments on Eau Claire shale and a review of relevant literature. International Journal of Greenhouse Gas Control, 7(2), 153–167.
Liu, J.-F., Sun, X.-B., Yang, G.-C., Mbadinga, S. M., Gu, J.-D., & Mu, B.-Z. (2015). Analysis of microbial communities in the oil reservoir subjected to CO2-flooding by using functional genes as molecular biomarkers for microbial CO2 sequestration. Frontiers in Microbiology. https://doi.org/10.3389/fmicb.2015.00236.
Mcglade, C., & Ekins, P. (2015). The geographical distribution of fossil fuels unused when limiting global warming to 2 °C. Nature, 517(7533), 187–190.
Merja, I., Mari, N., Anu, K., Aura, N., Lasse, A., & Ilmo, K. (2011). Characterization of bacterial diversity to a depth of 1500 m in the Outokumpu deep borehole, Fennoscandian Shield. FEMS Microbiology Ecology, 2, 295–309.
Mitchell, A. C., Phillips, A., Schultz, L., Parks, S., Spangler, L., Cunningham, A. B., et al. (2013). Microbial CaCO3 mineral formation and stability in an experimentally simulated high pressure saline aquifer with supercritical CO2. International Journal of Greenhouse Gas Control, 15(6), 86–96.
Morozova, D., Wandrey, M., Alawi, M., Zimmer, M., Vieth, A., Zettlitzer, M., et al. (2010). Monitoring of the microbial community composition in saline aquifers during CO storage by fluorescence in situ hybridisation. International Journal of Greenhouse Gas Control, 4(6), 981–989.
Mu, A., Boreham, C., Leong, H. X., Haese, R. R., & Moreau, J. W. (2014). Changes in the deep subsurface microbial biosphere resulting from a field-scale CO2 geosequestration experiment. Frontiers in Microbiology, 5(5), 209.
Mu, A., & Moreau, J. W. (2015). The geomicrobiology of CO2 geosequestration: A focused review on prokaryotic community responses to field-scale CO2 injection. Frontiers in Microbiology, 6(263), 263.
Page, D. I., Hickey, K. L., Narula, R., Main, A. L., & Grimberg, S. J. (2008). Modeling anaerobic digestion of dairy manure using the IWA Anaerobic Digestion Model no. 1 (ADM1). Water Science and Technology, 58(3), 689–695. https://doi.org/10.2166/wst.2008.678.
Pearce, J. K., Dawson, G. K. W., Golab, A., Knuefing, L., Sommacal, S., Rudolph, V., et al. (2019). A combined geochemical and μCT study on the CO2 reactivity of Surat Basin reservoir and cap-rock cores: Porosity changes, mineral dissolution and fines migration. International Journal of Greenhouse Gas Control, 80, 10–24.
Pruess, K., & Spycher, N. (2007). ECO2N-A fluid property module for the TOUGH2 code for studies of CO2 storage in saline aquifers. Energy Conversion and Management, 48(6), 1761–1767. https://doi.org/10.1016/j.enconman.2007.01.016.
Rathnaweera, T. D., Ranjith, P. G., Perera, M. S. A., Ranathunga, A. S., Wanniarachchi, W. A. M., Yang, S. Q., et al. (2017). An experimental investigation of coupled chemico-mineralogical and mechanical changes in varyingly-cemented sandstones upon CO2 injection in deep saline aquifer environments. Energy, 133, 404–414.
Rosenbauer, R. J., Koksalan, T., & Palandri, J. L. (2005). Experimental investigation of CO2–brine–rock interactions at elevated temperature and pressure: Implications for CO2 sequestration in deep-saline aquifers. Fuel Processing Technology, 86(14), 1581–1597.
Sanna, A., Uibu, M., Caramanna, G., Kuusik, R., & Maroto-Valer, M. M. (2015). ChemInform abstract: A review of mineral carbonation technologies to sequester CO2. Cheminform, 46(6), 8049–8080.
Şengör, S. S., Spycher, N. F., Ginn, T. R., Sani, R. K., & Peyton, B. (2007). Biogeochemical reactive–diffusive transport of heavy metals in Lake Coeur d’Alene sediments. Applied Geochemistry, 22(12), 2569–2594.
Shi, E., Li, J., & Zhang, M. (2019). Application of IWA Anaerobic Digestion Model No. 1 to simulate butyric acid, propionic acid, mixed acid, and ethanol type fermentative systems using a variable acidogenic stoichiometric approach. Water Research, 161, 242–250. https://doi.org/10.1016/j.watres.2019.05.094.
Vilcáez, J. (2015). Numerical modeling and simulation of microbial methanogenesis in geological CO2 storage sites. Journal of Petroleum Science and Engineering, 135, 583–595.
Vilcáez, J. (2020). Reactive transport modeling of produced water disposal into dolomite saline aquifers: Controls of barium transport. Journal of Contaminant Hydrology, 103600, 1529.
Vornolt, J., Kunow, J., Stetter, K. O., & Thauer, R. K. (1995). Enzymes and coenzymes of the carbon monoxide dehydrogenase pathway for autotrophic CO2 fixation in Archaeoglobus lithotrophicus and the lack of carbon monoxide dehydrogenase in the heterotrophic A. profundus. Archives of Microbiology, 163(2), 112–118.
Wan, Y., Du, S., Lin, G., Zhang, F., & Xu, T. (2017). Dissolution sequestration mechanism of CO2 at the Shiqianfeng saline aquifer in the Ordos Basin, northwestern China. Arabian Journal of Geosciences, 10(3), 71.
Wang, K., Xu, T., Tian, H., & Wang, F. (2016). Impacts of mineralogical compositions on different trapping mechanisms during long-term CO2 storage in deep saline aquifers. Acta Geotechnica, 11(5), 1–22.
Wigand, M., Carey, J. W., Schütt, H., Spangenberg, E., & Erzinger, J. (2008). Geochemical effects of CO sequestration in sandstones under simulated in situ conditions of deep saline aquifers. Applied Geochemistry, 23(9), 2735–2745.
Woo, S. H., & Rittmann, B. E. (2000). Microbial energetics and stoichiometry for biodegradation of aromatic compounds involving oxygenation reactions. Biodegradation, 11(4), 213.
Xiao, J., & Vanbriesen, J. M. (2010). Expanded thermodynamic model for microbial true yield prediction. Biotechnology and Bioengineering, 93(1), 110–121.
Xu, T. (2001). Modeling multiphase non-isothermal fluid flow and reactive geochemical transport in variably saturated fractured rocks: 1. Methodology. American Journal of Science, 301(1), 16–33.
Xu, T. (2008). Incorporating aqueous reaction kinetics and biodegradation into TOUGHREACT: Applying a multiregion model to hydrobiogeochemical transport of denitrification and sulfate reduction. Vadose Zone Journal, 7(1), 305–315.
Xu, T., Apps, J. A., Pruess, K., & Yamamoto, H. (2007). Numerical modeling of injection and mineral trapping of CO2 with H2S and SO2 in a sandstone formation. Chemical Geology, 242(3), 319–346.
Xu, T., Sonnenthal, E., Spycher, N., & Pruess, K. (2006). Toughreact—A simulation program for non-isothermal multiphase reactive geochemical transport in variably saturated geologic media: Applications to geothermal injectivity and CO2 geological sequestration. Computers and Geosciences, 32(2), 145–165.
Yang, Z., Xu, T., Wang, F., Yang, Y., Li, X., & Zhao, N. (2018). Impact of inner reservoir faults on migration and storage of injected CO2. International Journal of Greenhouse Gas Control, 72, 14–25.
Yu, X. R., Ahmadinia, M., Shariatipour, S. M., Lawton, D., Osadetz, K., & Saeedfar, A. (2020). Impact of reservoir permeability, permeability anisotropy and designed injection rate on CO(2) gas behavior in the shallow saline aquifer at the CaMI field research station, Brooks, Alberta. Natural Resources Research, 29(4), 2735–2752. https://doi.org/10.1007/s11053-019-09604-3.
Zhang, K., Wu, Y. S., & Pruess, K. (2008). User’s guide for TOUGH2-MP-a massively parallel version of the TOUGH2 code. Lawrence Berkeley National Laboratory.
Zheng, F., Shi, X. Q., Wu, J. C., Chen, Y., & Xu, H. X. (2013). Global sensitivity analysis of reactive transport modeling of CO2 geological storage in a saline aquifer. Procedia Earth & Planetary Science, 7, 798–801.
Acknowledgments
This work was supported by biodegradation Environment of Petroleum hydrocarbons in shallow pore groundwater (Grant number 41402207). We thank Lucy Muir, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, X., Dong, W., Wan, Y. et al. Numerical Simulation of Effects of Microbial Action on CO2 Geological Storage in Deep Saline Aquifers. Nat Resour Res 30, 1629–1648 (2021). https://doi.org/10.1007/s11053-020-09780-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11053-020-09780-7