Abstract
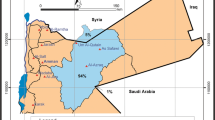
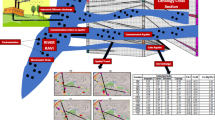
Sophisticated geochemical models have been used to describe and predict the chemical behaviour of complex natural waters and also to protect the groundwater resources from future contamination. One such model is used to study the hydrogeochemical complexity in a mine area. Extraction of groundwater from the coastal aquifer has been in progress for decades to mine lignite in Neyveli. This extraction has developed a cone of depression around the mine site. This cone of depression is well established by the geochemical nature of groundwater in the region. 42 groundwater samples were collected in a definite pattern and they were analysed for major cations, anions and trace elements. The saturation index (SI) of the groundwater for carbonate, sulphate and silica minerals was studied and it has been correlated with the recharge and the discharge regions. The SI of alumino silicates has been used to decipher the stage of weathering. The SIGibbsite − SIK-feldspar has been spatially distributed and the regions of discharge and recharge were identified. Then two flow paths A1 and A2 were identified and inverse modelling using PHREEQC were carried out to delineate the geochemical process that has taken place from recharge to discharge. The initial and final solutions in both the flow paths were correlated with the thermodynamic silicate stability diagrams of groundwater and it was found that the state of thermodynamic stability of the end solutions along the flow path were approaching similar states of equilibrium at the discharge.











Similar content being viewed by others
References
Anandhan, P. (2005). Hydrogeochemical studies in and around Neyveli mining region, Tamil Nadu, India. Ph.D. Thesis, Department of Earth Sciences, Annamalai University, 189 pp.
APHA. (1995). Standard methods for the examination of water and wastewater (19th ed.). Washington, DC: APHA.
CGWB. (1992). Report of the working group on the estimation of ground resources and irrigation potential of Periyar district, Tamil Nadu (Unpublished Report).
Chidambaram, S. (2000). Hydrogeochemical studies of groundwater in Periyar district, Tamil Nadu, India. Unpublished Ph.D. Thesis, Department of Geology, Annamalai University, India.
Deutsch, W. J. (1997). Ground water geochemistry: Fundamentals and application to contamination. Boca Raton: Lewis Publishers.
Drever, I. J. (1988). The geochemistry of natural waters (2nd ed., 388 pp.). Englewood Cliffs, NJ: Prentice Hall.
Elangovan, K. (1997). Hydrogeochemistry of groundwater forming heavy scales in water supply system of Salem district, Tamil Nadu (334 pp.). Unpublished Ph.D. Thesis, Mysore University, Mysore.
Elangovan, K., Balasubramanian, A., Thirugnana Sambandam, R., Rengarajan, R., Satish, P. N., & Janardhan, A. S. (1999). Geochemical modeling of crystalline aquifers of Salem district, Tamil Nadu. Indian J Geochem, 14, 1–17.
Felmy, A. R., Bervin, D. C., & Jenne, E. A. (1984) MINTEQ. A computer program for calculating aqueous geochemical equilibria. US EPA 84-032, Athens GA.
Garrels, R. M., & Christ, C. L. (1965). Solutions minerals and equilibria (450 pp.). New York: Harper and Row.
Gibbs, R. J. (1970). Mechanisms controlling world’s water chemistry. Science, 170, 1088–1090.
Gowrishanker, S., Meyyappan, M., & Radhakrishnan, M. (1980). Lignite deposits and groundwater development on Neyveli area. Bullet. ONGC, 17, 1–16.
Helgeson, H. C., Brown, T. H., Nigrini, A., & Jones, T. A. (1970). Calculation of mass transfer in geochemical processes involving aqueous solutions. Geochimica et Cosmochimica Acta, 34, 569–592.
Jenne, E. A. (1981). Geochemical modeling: A review waste Rock interactions program. Pacific North West laboratory Report 3574: VC70, 47 pp.
Konrad, B. K. (1983). Introduction to geochemistry (2nd ed.). New York: McGraw-Hill International Book Company.
Murphy, E. M., & Schramke, J. A. (1998). Estimation of microbial respiration rates in groundwater by geochemical modeling constrained with stable isotopes. Geochimica et Cosmochimica Acta, 62(21/22), 3395–3406.
Nordstrom, D. K., Plummer, L. N., Wigley, T. M. L., Wolery, T. J., Ball, J. W., Jenne, E. A., et al. (1979). A comparison of computerized chemical models for equilibrium calgllations in aqueous systems. In E. A. Jenne (Ed.), Chemical modeling in aqueous systems (pp. 857–892). Washington, DC: American Chemical Society, Symposium Series 93.
Parkhurst, DL., Thorstenson, DC., & Plummer, LN. (1990) PHREEQE—A computer program for geochemical calculation. USGS water resources investigations report 80-96, 195 pp.
Plummer, L. N., Busby, J. F., Lee, R. W., & Hanshaw, B. B. (1990). Geochemical modeling of the madison aquifer in parts of Montana, Wyoming and South Dakota. Water Resources Research, 26, 1981–2014.
Plummer, L. N., Jones, B. F., & Truesdell, A. H. (1976). WATEQF—A fortran IV version of water, a computer program for calculating chemical equilibrium of natural waters. US Geol Survey water resources investigations report 76, 1361 pp.
Plummer, L. N., Parkhurst, D. L., & Thorstenson, D. C. (1983). Development of reaction models for groundwater systems. Geochimica et Cosmochimica Acta, 47, 665–685.
Prasanna, M. V., Chidambaram, S., Srinivasamoorthy, K., Anandhan, P., & John Peter, A. (2006). A study on hydrogeochemistry along the ground water flow path in different litho units in Gadilam river basin, Tamil Nadu (India). International Journal of Chemical Sciences, 2(2), 157–172.
Ramanathan, A. L. (1992). Geochemical studies in the Cauvery river basin. Unpublished Ph.D. Thesis. Punjab University, Chandigarh, India.
Ramesh, R., & Anbu, M. (1996). Chemical methods for environmental analysis: Water and sediment (161 pp.) Madras: Macmillan India Ltd.
Runnels, D. D. (1978). American research in the geochemical modeling of groundwater. Journal of the Geological Society of India, 29, 135–144.
Sanford, W. E., & Konikow, L. F. (1989). Simulation of calcite dissolution and porosity change to in salt-water mixing zones in coastal aquifers. Water Resources Research, 25, 655–667.
Shannon, D. W., Morray, J. R., & Smith, P. R. (1977). Use of a chemical equilibrium model computer code to analyze scale formation and corrosion in geothermal brines. Proceedings in Symposium on Oil Field and Geothermal Chemistry Conference 770609, pp. 21–36.
Spostigo, G., & Mattigod, S. V. (1979). GEOCHEM: A computer program for the calculation of chemical equlibria in soil solution and other natural water systems. Riverside, CA: Department of Soil and Environmental Sciences, University of California.
Stumm, W., & Morgan, J. J. (1996). Aquatic chemistry (p. 1022 pp). New York: John Wiley and Sons Inc.
Tamata, S. R. (1990). Mineral-CaCO3 saturation and stability of groundwater in Kamataka state—A preliminary study. Bhu-Jal News 8–384, 14–24.
Truesdell, A. H., & Jones, B. F. (1973). WATEQ: A computer program for calculating chemical equilibria of natural waters. Journal of Research of the U.S. Geological Survey, 2(2), 233–248.
Uma Maheswaran, T. (2007) Hydrogeochemical modeling of in and around Devakottai region, Tamil Nadu. M.Phil. Thesis, Department of Earth Sciences, Annamalai University.
Wolery, T. J. (1979). Calculation of chemical equilibrium between aqueous solutions and minerals: The EQ3/EQ6 software package (41 pp.). Livermore, University of California, Lawrence Livermore Laboratory (UCRL 52658).
Wolery, T. J. (1983). EQ3NR: A computer program for geochemical aqueous specification—Solubility calculations (191 pp.). Livermore, California: User’s Guide and documentation, Lawrence Livermore National Lab UCRL-53414.
Zhu, C., & Anderson, C. (2002). Environmental applications of geochemical modeling. Cambridge: Cambridge University Press.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chidambaram, S., Anandhan, P., Prasanna, M.V. et al. Hydrogeochemical Modelling for Groundwater in Neyveli Aquifer, Tamil Nadu, India, Using PHREEQC: A Case Study. Nat Resour Res 21, 311–324 (2012). https://doi.org/10.1007/s11053-012-9180-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11053-012-9180-6