Abstract
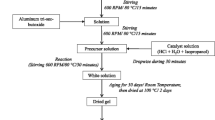
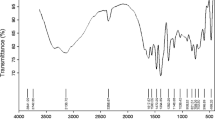
MgAl2O4 spinel nanoparticles were successfully synthesized using the polyol-mediated process. The study focused on the use of aluminum nitrate and magnesium acetate as precursor salts, with diethylene glycol as the solvent. The synthesis was conducted at three different temperatures: 150, 180, and 230 °C, followed by a subsequent calcination step. The dried samples consisted of a combination of magnesium oxalate and an unidentified amorphous or nanostructured phase. Upon calcination, all samples exhibited the desired MgAl2O4 spinel structure, along with a small amount of MgO. The FTIR spectra of the calcined samples confirmed the crystal structure of the MgAl2O4 phase as an inverse spinel. Scanning electron microscopy images revealed the quasi-spherical morphology of the nanoparticles, with particle sizes ranging from 70 to 120 nm for dried samples and 50 to 60 nm for calcined samples.






Similar content being viewed by others
References
Ganesh I (2013) A review on magnesium aluminate (MgAl2O4) spinel: synthesis, processing and applications. Int Mater Rev 58(2):63–112. https://doi.org/10.1179/1743280412Y.0000000001
Lv L, Xiao G, Ding D (2021) Improved thermal shock resistance of low-carbon Al2O3–C refractories fabricated with C/MgAl2O4 composite powders. Ceram Int 47(14):20169–20177. https://doi.org/10.1016/J.CERAMINT.2021.04.023
Shafiee H, Salehirad A, Samimi A (2020) Effect of synthesis method on structural and physical properties of MgO/MgAl2O4 nanocomposite as a refractory ceramic. Appl Phys A Mater Sci Process 126(3). https://doi.org/10.1007/s00339-020-3369-z
Gu Q, Zhao F, Liu X, Jia Q (2019) Preparation and thermal shock behavior of nanoscale MgAl2O4 spinel-toughened MgO-based refractory aggregates. Ceram Int 45(9):12093–12100. https://doi.org/10.1016/J.CERAMINT.2019.03.107
Gajdowski C et al (2017) Influence of post-HIP temperature on microstructural and optical properties of pure MgAl2O4 spinel: from opaque to transparent ceramics. J Eur Ceram Soc 37(16):5347–5351. https://doi.org/10.1016/J.JEURCERAMSOC.2017.07.031
Balabanov SS et al (2015) Fabrication of transparent MgAl2O4 ceramics by hot-pressing of sol-gel-derived nanopowders. Ceram Int 41(10):13366–13371. https://doi.org/10.1016/J.CERAMINT.2015.07.123
Zhang P et al (2015) Aqueous gelcasting of the transparent MgAl2O4 spinel ceramics. J Alloys Compd 646:833–836. https://doi.org/10.1016/J.JALLCOM.2015.05.275
Yu S, Hu Y, Cui H, Cheng Z, Zhou Z (2021) Ni-based catalysts supported on MgAl2O4 with different properties for combined steam and CO2 reforming of methane. Chem Eng Sci 232:116379. https://doi.org/10.1016/J.CES.2020.116379
Rezaei M, Alavi SM (2019) Dry reforming over mesoporous nanocrystalline 5% Ni/M-MgAl2O4 (M: CeO2, ZrO2, La2O3) catalysts. Int J Hydrogen Energy 44(31):16516–16525. https://doi.org/10.1016/J.IJHYDENE.2019.04.213
Qiu Y, Fu E, Gong F, Xiao R (2022) Catalyst support effect on ammonia decomposition over Ni/MgAl2O4 towards hydrogen production. Int J Hydrogen Energy 47(8):5044–5052. https://doi.org/10.1016/J.IJHYDENE.2021.11.117
Wongtawee W, Amornpitoksuk P, Randorn C, Rattana T, Suwanboon S (2022) Photocatalytic activity under visible light illumination of organic dyes over g-C3N4/MgAl2O4 nanocomposite. J Indian Chem Soc 99(8):100628. https://doi.org/10.1016/J.JICS.2022.100628
Wongtawee W, Amornpitoksuk P, Randorn C, Rattana T, Suwanboon S (2023) Amelioration of photocatalytic activity of MgAl2O4 spinel photocatalyst by coupling with WO3. Inorg Chem Commun 152:110654. https://doi.org/10.1016/J.INOCHE.2023.110654
Riahi R, Taher MA, Beitollahi H (2022) Hydroxylamine electrochemical sensor based on magnesium aluminate spinel nanoparticles modified electrode. Int J Environ Anal Chem 1–13. https://doi.org/10.1080/03067319.2022.2118587
Li X et al (2022) Insight into site occupancy of cerium and manganese ions in MgAl2O4 and their energy transfer for dual-mode optical thermometry. J Alloys Compd 928:166701. https://doi.org/10.1016/J.JALLCOM.2022.166701
Hatton P, Uberuaga BP (2023) Short range order in disordered spinels and the impact on cation vacancy transport. J Mater Chem A Mater 11(7):3471–3480. https://doi.org/10.1039/D2TA06102C
Lepkova D, Batarjav A, Samuneva B, Ivanova LGY (1991) Preparation and properties of ceramics from magnesium spinel by sol-gel technology. J Mater Sci 26(4861):4864
Yuan Y, Zhang S, You W (2004) Synthesis of MgAl2O4 spinel nanometer powder via biology polysaccharide assisted sol-gel process. J Solgel Sci Technol 30:223–227
Sanjabi S, Obeydavi A (2015) Synthesis and characterization of nanocrystalline MgAl2O4 spinel via modified sol–gel method. J Alloys Compd 645:535–540. https://doi.org/10.1016/j.jallcom.2015.05.107
Zarazúa-Villalobos L, Téllez-Jurado L, Vargas-Becerril N, Fantozzi G, Balmori-Ramírez H (2015) Synthesis of magnesium aluminate spinel nanopowder by sol–gel and low-temperature processing. J Solgel Sci Technol 85(1):110–120. https://doi.org/10.1007/s10971-017-4526-5
Khomidov FG, Kadyrova ZR, Usmanov KhL, Niyazova ShM, Sabirov BT (2021) Peculiarities of sol-gel synthesis of aluminum-magnesium spinel. Glass Ceram 78(5–6):251–254. https://doi.org/10.1007/s10717-021-00389-7
Li J-G, Ikegami T, Lee J-H, Mori T, Yajima Y (2001) A wet-chemical process yielding reactive magnesium aluminate spinel (MgAl2O4) powder. Ceram Int 27(4):481–489. https://doi.org/10.1016/S0272-8842(00)00107-3
Zawrah MF, Hamaad H, Meky S (2007) Synthesis and characterization of nano MgAl2O4 spinel by the co-precipitated method. Ceram Int 33(6):969–978. https://doi.org/10.1016/j.ceramint.2006.02.015
Wajler A, Tomaszewski H, Drożdż-Cieśla E, Węglarz H, Kaszkur Z (2008) Study of magnesium aluminate spinel formation from carbonate precursors. J Eur Ceram Soc 28(13):2495–2500. https://doi.org/10.1016/j.jeurceramsoc.2008.03.013
Rashad MM, Zaki ZI, El-Shall H (2009) A novel approach for synthesis of nanocrystalline MgAl2O4 powders by co-precipitation method. J Mater Sci 44(11):2992–2998. https://doi.org/10.1007/s10853-009-3397-8
Rufner J, Anderson D, van Benthem K, Castro RHR (2013) Synthesis and sintering behavior of ultrafine (<10 nm) magnesium aluminate spinel nanoparticles. J Am Ceram Soc 96(7):2077–2085. https://doi.org/10.1111/jace.12342
Suyama Y, Kato A (1982) Characterization and sintering of Mg Al spinel prepared by spray-pyrolysis technique. Ceram Int 8(1):17–21. https://doi.org/10.1016/0272-8842(82)90010-4
Bickmore CR, Waldner KF, Treadwell DR, Laine RM (1996) Ultrafine spinel powders by flame spray pyrolysis of a magnesium aluminum double alkoxide. J Am Ceram Soc 79(5):1419–1423. https://doi.org/10.1111/j.1151-2916.1996.tb08608.x
Tong Y, Zhao S, Ma L, Zhao W, Song W, Yang H (2013) Facile synthesis and crystal growth dynamics study of MgAl2O4 nanocrystals. Mater Res Bull 48(11):4834–4838. https://doi.org/10.1016/j.materresbull.2013.08.039
Sarkar R, Das S (2014) Auto combustion synthesis for magnesium aluminate spinel using glycine as fuel and its sintering study. Trans Indian Ceram Soc 73(2):172–176. https://doi.org/10.1080/0371750X.2014.922436
Baruah B, Bhattacharyya S, Sarkar R (2023) Synthesis of magnesium aluminate spinel—an overview. Int J Appl Ceram Technol 20(3):1331–1349. https://doi.org/10.1111/ijac.14309
Fiévet F, Brayner R (2013) “The polyol process”, in Nanomaterials: a danger or a promise? London Springer, London 1:25. https://doi.org/10.1007/978-1-4471-4213-3_1
Fievet F, Lagier JP, Blin B, Beaudoin B, Figlarz M (1989) Homogeneous and heterogeneous nucleations in the polyol process for the preparation of micron and submicron size metal particles. Solid State Ion 32–33(PART 1):198–205. https://doi.org/10.1016/0167-2738(89)90222-1
Jézéquel D, Guenot J, Jouini N, Fiévet F (1995) Submicrometer zinc oxide particles: elaboration in polyol medium and morphological characteristics. J Mater Res 10(1):77–83. https://doi.org/10.1557/JMR.1995.0077
Fiévet F et al (2018) The polyol process: a unique method for easy access to metal nanoparticles with tailored sizes, shapes and compositions. Chem Soc Rev 47(14):5187–5233. https://doi.org/10.1039/C7CS00777A
Ahmed KM, McLeod MP, Nézivar J, Giuliani AW (2010) Fourier transform infrared and near-infrared spectroscopic methods for the detection of toxic diethylene glycol (DEG) contaminant in glycerin based cough syrup. Spectroscopy 24(6):601–608. https://doi.org/10.3233/SPE-2010-0482
Shou-Yong J, Li-Bin L, Ning-Kang H, Jin Z, Yong L (2000) Investigation on lattice constants of Mg-Al spinels. J Mater Sci Lett 19(3):225–227. https://doi.org/10.1023/A:1006710808718
Erukhimovitch V, Mordekoviz Y, Hayun S (2015) Spectroscopic study of ordering in non-stoichiometric magnesium aluminate spinel. Am Miner 100(8–9):1744–1751. https://doi.org/10.2138/am-2015-5266
Funding
This research has received support from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—finance code 88887.464399/2019–00 and the Conselho Nacional de Desenvolvimento Científico e Tecnológico—Brasil (CNPq)—finance code 313915/2021–0.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lopes Nunes Abreu dos Santos, P.H., Ribeiro, S. Polyol-mediated synthesis and characterization of magnesium–aluminum spinel nanoparticles. J Nanopart Res 26, 37 (2024). https://doi.org/10.1007/s11051-024-05953-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-024-05953-0