Abstract
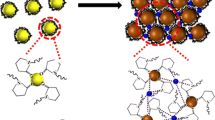
Current work presents a detailed study on the synthesis of silver nanoparticles (70 ± 5 nm) functionalized with 4-mercaptobenzoic acid and their interaction with divalent ions (Cu2+, Fe2+, Co2+, Ni2+, Pb2+, Mn2+, Zn2+, Sn2+). The study reveals the morphological changes in the nanoparticles due to interaction with Cu2+, resulting in the formation of larger nanoparticle aggregates. The UV-Vis spectra of the pristine and aggregated nanoparticles are also discussed, providing insights into the changes in their optical properties. These nanoparticles are used to detect divalent ions using surface-enhanced Raman spectroscopy with a detection limit of 2.5 × 10–7 M for Cu2+ and also show a linear response within the 10–3 to 10–7 M concentration range. High selectivity for Cu2+, Fe2+, Co2+, Mn2+, and Pb2+ was also detected. The findings of this study could have significant implications in the field of nanomaterials and environmental chemistry, particularly in the detection and removal of heavy metal ions.








Similar content being viewed by others
References
Kolahalam LA, Kasi Viswanath IV, Diwakar BS, Govindh B, Reddy V, Murthy YLN (2019) Review on nanomaterials: synthesis and applications. Mater Today Proc 18:2182–2190. https://doi.org/10.1016/j.matpr.2019.07.371
Baig N, Kammakakam I, Falath W, Kammakakam I (2021) Nanomaterials: a review of synthesis methods, properties, recent progress, and challenges. Mater Adv 2(6):1821–1871. https://doi.org/10.1039/d0ma00807a
Willner MR, Vikesland PJ (2018) Nanomaterial enabled sensors for environmental contaminants. J Nanobiotech 16(1):1–16. https://doi.org/10.1186/s12951-018-0419-1
Li Y, Wang Z, Sun L, Liu L, Xu C, Kuang H (2019) Nanoparticle-based sensors for food contaminants. TrAC - Trends Anal Chem 113:74–83. https://doi.org/10.1016/j.trac.2019.01.012
Ganesh KM, Rai A, Battampara P et al (2023) Optical coupling of bio-inspired mustard protein-based bimetallic nanohybrids with propagating surface plasmon polaritons for femtomolar nitrite ion sensing: cellphone-based portable detection device. Nano-Struct and Nano-Objects 35:101025. https://doi.org/10.1016/j.nanoso.2023.101025
Bhaskar S, Singh AK, Das P et al (2020) Superior resonant nanocavities engineering on the photonic crystal-coupled emission platform for the detection of femtomolar iodide and zeptomolar cortisol. ACS Appl Mater Interfaces 12(30):34323–34336. https://doi.org/10.1021/acsami.0c07515
Gall JE, Boyd RS, Rajakaruna N (2015) Transfer of heavy metals through terrestrial food webs: a review. Environ Monit Assess 187(4):201. https://doi.org/10.1007/s10661-015-4436-3
Zwolak A, Sarzyńska M, Szpyrka E, Stawarczyk K (2019) Sources of soil pollution by heavy metals and their accumulation in vegetables: a review. Water Air Soil Pollut 230(7):164. https://doi.org/10.1007/s11270-019-4221-y
Malik LA, Bashir A, Qureashi A, Pandith AH (2019) Detection and removal of heavy metal ions: a review. Environ Chem Lett 17(4):1495–1521. https://doi.org/10.1007/s10311-019-00891-z
Bolisetty S, Peydayesh M, Mezzenga R (2019) Sustainable technologies for water purification from heavy metals: review and analysis. Chem Soc Rev 48(2):463–487. https://doi.org/10.1039/c8cs00493e
Langer J, de Aberasturi DJ, Aizpurua J et al (2020) Present and future of surface-enhanced Raman scattering. ACS Nano 14(1):28–117. https://doi.org/10.1021/acsnano.9b04224
Betz JF, Yu WW, Cheng Y, White IM, Rubloff GW (2014) Simple SERS substrates: powerful, portable, and full of potential. Phys Chem Chem Phys 16(6):2224–2239. https://doi.org/10.1039/c3cp53560f
Mosier-Boss PA (2017) Review of SERS substrates for chemical sensing. Nanomaterials 7(6):142. https://doi.org/10.3390/nano7060142
Li Z, Huang X, Lu G (2020) Recent developments of flexible and transparent SERS substrates. J Mater Chem C 8(12):3956–3969. https://doi.org/10.1039/d0tc00002g
Pilot R, Signorini R, Durante C, Orian L, Bhamidipati M, Fabris L (2019) A review on surface-enhanced Raman scattering. Biosensors 9(2):57. https://doi.org/10.3390/bios9020057
Alvarez-Puebla RA, Liz-Marzán LM (2012) SERS detection of small inorganic molecules and ions. Angew Chem Int Ed 51(45):11214–11223. https://doi.org/10.1002/anie.201204438
Xu G, Zhang Q, Gao C, Ma L, Song P, Xia L (2021) A label-free SERS sensor for the detection of Hg2+ based on phenylacetylene functionalized Ag nanoparticles. Microchem J 168:106504. https://doi.org/10.1016/j.microc.2021.106504
Qi L, Xiao M, Wang F et al (2017) Poly-cytosine-mediated nanotags for SERS detection of Hg2+. Nanoscale 9(37):14184–14191. https://doi.org/10.1039/c7nr05165d
Eeparuksapun KT, Rasongchan NP, Hawonsuwan AT (2019) Alpha-lipoic acid- functionalized silver nanoparticles for colorimetric detection of copper ion. Anal Sci 35:371–377. https://doi.org/10.2116/analsci.18P442
Wei J, Chen J, Yue G et al (2018) Development of a novel tridentate ligand for colorimetric detection of Mn2+ based on Ag NPs. Spectrochim Acta - Part A Mol Biomol Spectrosc 202:244–251. https://doi.org/10.1016/j.saa.2018.05.033
Tan E, Yin P, Lang X, Zhang H, Guo L (2012) A novel surface-enhanced Raman scattering nanosensor for detecting multiple heavy metal ions based on 2-mercaptoisonicotinic acid functionalized gold nanoparticles. Spectrochim Acta - Part A Mol Biomol Spectrosc 97:1007–1012. https://doi.org/10.1016/j.saa.2012.07.114
Sharma S, Jaiswal A, Uttam KN (2022) Determination of chromium(VI), chromium(III), arsenic(V), aluminum(III), iron(II), and manganese(II) by colorimetry and surface-enhanced raman scattering (SERS) using ferulic acid functionalized silver nanoparticles. Anal Lett 55(5):715–727. https://doi.org/10.1080/00032719.2021.1963269
Devi NR, Sasidharan M, Sundramoorthy AK (2018) Gold nanoparticles-thiol-functionalized reduced graphene oxide coated electrochemical sensor system for selective detection of mercury ion. J Electrochem Soc 165(8):B3046–B3053. https://doi.org/10.1149/2.0081808jes
Chen D, Li J (2006) Interfacial design and functionization on metal electrodes through self-assembled monolayers. Surf Sci Rep 61(11):445–463. https://doi.org/10.1016/j.surfrep.2006.08.001
Li XM, Huskens J, Reinhoudt DN (2004) Reactive self-assembled monolayers on flat and nanoparticle surfaces, and their application in soft and scanning probe lithographic nanofabrication technologies. J Mater Chem 14(20):2954–2971. https://doi.org/10.1039/b406037g
Vericat C, Vela ME, Corthey G et al (2014) Self-assembled monolayers of thiolates on metals: a review article on sulfur-metal chemistry and surface structures. RSC Adv 4(53):27730–27754. https://doi.org/10.1039/c4ra04659e
Garg N, Carrasquillo-Molina E, Lee TR (2002) Self-assembled monolayers composed of aromatic thiols on gold: structural characterization and thermal stability in solution. Langmuir 18(7):2717–2726. https://doi.org/10.1021/la0115278
Kiran Bharti R, Sharma R (2021) Effect of heavy metals: an overview. Mater Today Proc 51:880–885. https://doi.org/10.1016/j.matpr.2021.06.278
Singh J, Kalamdhad AS (2011) Effects of heavy metals on soil, plants, human health and aquatic life. Int J Res Chem Environ 1(2):15–21 https://www.ijrce.org/index.php/ijrce/article/view/78/65. Accessed 24 Dec 2023
Rehman M, Liu L, Wang Q et al (2019) Copper environmental toxicology, recent advances, and future outlook: a review. Environ Sci Pollut Res 26:18003–18016. https://doi.org/10.1007/s11356-019-05073-6
Drinking Water Requirements for States and Public Water Systems, Lead and Copper Rule, United States Environmental Protection Agency. https://www.epa.gov/dwreginfo/lead-and-copper-rule. Accessed 16 Oct 2023
Bala T, Prasad BLV, Sastry M, Kahaly MU, Waghmare UV (2007) Interaction of different metal ions with carboxylic acid group: a quantitative study. J Phys Chem A 111(28):6183–6190. https://doi.org/10.1021/jp067906x
Salleh A, Naomi R, Utami ND et al (2020) The potential of silver nanoparticles for antiviral and antibacterial applications: a mechanism of action. Nanomaterials 10(8):1566. https://doi.org/10.3390/nano10081566
Mukherji S, Bharti S, Shukla G, Mukherji S (2019) Synthesis and characterization of size- and shape-controlled silver nanoparticles. Phys Sci Rev 4(1):2170082. https://doi.org/10.1515/psr-2017-0082
Celis F, Campos-Vallette M, Vega JC, Gómez-Jeria JS, Aliaga CJ (2015) Raman and surface enhanced Raman signals of the sensor 1-(4-mercaptophenyl)-2,4,6-triphenylpyridinium perchlorate. Chil Chem Soc 60(2):2944–2948. https://doi.org/10.4067/S0717-97072015000200018
Kong X, Yu Q, Zhang X, Du X, Gong H, Jiang H (2012) Synthesis and application of surface enhanced Raman scattering (SERS) tags of Ag@SiO2 core/shell nanoparticles in protein detection. J Mater Chem 22(16):7767–7774. https://doi.org/10.1039/c2jm16397g
Zhou Y, Zhao H, He Y, Ding N, Cao Q (2011) Colorimetric detection of Cu2+ using 4-mercaptobenzoic acid modified silver nanoparticles. Colloids Surfaces A Physicochem Eng Asp 391(1-3):179–183. https://doi.org/10.1016/j.colsurfa.2011.07.026
Ho CH, Lee S (2015) SERS and DFT investigation of the adsorption behavior of 4-mercaptobenzoic acid on silver colloids. Colloids Surfaces A Physicochem Eng Asp 474:29–35. https://doi.org/10.1016/j.colsurfa.2015.03.004
Handa S, Yu Y, Futamata M (2014) Adsorbed state of p-mercaptobenzoic acid on silver nanoparticles. Vib Spectrosc 72:128–133. https://doi.org/10.1016/j.vibspec.2014.03.007
Michota A, Bukowska J (2003) Surface-enhanced Raman scattering (SERS) of 4-mercaptobenzoic acid on silver and gold substrates. J Raman Spectrosc 34(1):21–25. https://doi.org/10.1002/jrs.928
Daublytė E, Zdaniauskienė A, Talaikis M, Drabavičius A, Charkova T (2021) A facile microwave-assisted synthesis of Ag@SiO2 nanoparticles for Raman spectroscopy. New J Chem 45(24):10952–10958. https://doi.org/10.1039/d1nj01439k
Li R, Lv H, Zhang X et al (2015) Vibrational spectroscopy and density functional theory study of 4-mercaptobenzoic acid. Spectrochim Acta - Part A Mol Biomol Spectrosc 148:369–374. https://doi.org/10.1016/j.saa.2015.03.132
Rai A, Bhaskar S, Ganesh KM, Ramamurthy SS (2022) Hottest hotspots from the coldest cold: welcome to nano 4.0. ACS Appl Nano Mater 5(9):12245–12264. https://doi.org/10.1021/acsanm.2c02556
Ndokoye P, Ke J, Liu J, Zhao Q, Li X (2014) L -cysteine-modified gold nanostars for sers-based copper ions detection in aqueous media. Langmuir 30(44):13491–13497. https://doi.org/10.1021/la503553y
Acknowledgements
The authors acknowledge Audrius Drabavičius from the Department of Characterisation of Materials Structure of Center for Physical Sciences and Technology for HR-TEM analysis. The authors also thank Andrius Pakalniškis from the Faculty of Chemistry and Geosciences of Vilnius University for SEM analysis.
Author information
Authors and Affiliations
Contributions
Edita Daublytė: Investigation, Validation, Formal analysis. Agnė Zdaniauskienė: Data curation, Visualisation. Martynas Talaikis: Writing, Visualisation. Tatjana Charkova: Conceptualization, Methodology, Writing, Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Daublytė, E., Zdaniauskienė, A., Talaikis, M. et al. Synthesis and functionalization of silver nanoparticles for divalent metal ion detection using surface-enhanced Raman spectroscopy. J Nanopart Res 26, 6 (2024). https://doi.org/10.1007/s11051-023-05917-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-023-05917-w