Abstract
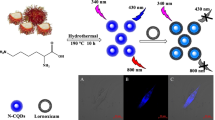
Carbon quantum dots (CQDs) with outstanding fluorescence properties have shown enormous applications in bioimaging due to their biocompatibility and nano size attribute. However, the toxicity of QDs may hinder their implementation in biomedical use. Here, nitrogen-doped carbon quantum dots (N-CQDs) were produced by employing a microwave-assisted approach and then their in vivo and in vitro toxicity has been investigated. An in vitro toxicity study was conducted against human breast adenocarcinoma cell lines (MDA-MB-361) by considering the concentration of 0–600μg mL−1 of the N-CQDs and the in vivo toxicity examination was done in Swiss albino mice for 30 days by considering two concentrations of N-CQDs, i.e., 10mg/kg BW (body weight) and 20mg/kg BW. Several parameters have been inspected by these studies like cell viability, antioxidant enzymes studies, and hematological and histopathological studies, and concluded that the synthesized NCQDs are non-toxic and can be used safely for bioimaging. Furthermore, the luminescence properties of N-CQDs were inquired by labeling them on a variety of fungal and bacterial cells. When the N-CQD-labeled cells were excited at two wavelengths, it leads to the emission of green and red fluorescence enabling them ideal for bioimaging. Briefly, there is a production of inexpensive and biocompatible N-CQDs of an average particle size of ~3.16 nm that can serve as universal fluorescent agents for the detection of diverse groups of pathogenic microorganisms.










Similar content being viewed by others
Data availability
The data supporting the study’s conclusions are accessible upon request from the corresponding author.
References
Sokolov K, Follen M, Aaron J et al (1999) Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res 63:1999–2004
Zhang L, Xing Y, He N et al (2012) Preparation of graphene quantum dots for bioimaging application. J Nanosci Nanotechnol 12:2924–2928. https://doi.org/10.1166/jnn.2012.5698
Santra S, Yang H, Dutta D et al (2004) TAT conjugated, FITC doped silica nanoparticles for bioimaging applications. Chem Commun 24:2810–2811. https://doi.org/10.1039/b411916a
Wolfbeis OS (2015) An overview of nanoparticles commonly used in fluorescent bioimaging. Chem Soc Rev 44:4743–4768
Smith AM, Nie S (2010) Semiconductor nanocrystals: structure, properties, and band gap engineering. Acc Chem Res 43:190–200. https://doi.org/10.1021/ar9001069
Michalet X, Pinaud FF, Bentolila LA et al (2005) Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307(5709):538–544
Medintz IL, Tetsuo Uyeda H, Goldman ER, Mattoussi H (2005) Quantum dot bioconjugates for imaging, labelling and sensing. Nat Mater 4(6):435–446
Smith AM, Duan H, Mohs AM, Nie S (2008) Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv Drug Deliv Rev 60:1226–1240
Arshad A, Akram R, Iqbal S et al (2019) Aqueous synthesis of tunable fluorescent, semiconductor CuInS2 quantum dots for bioimaging. Arab J Chem 12:4840–4847. https://doi.org/10.1016/j.arabjc.2016.10.002
Maestro LM, Ramírez-Hernández JE, Bogdan N et al (2012) Deep tissue bio-imaging using two-photon excited CdTe fluorescent quantum dots working within the biological window. Nanoscale 4:298–302. https://doi.org/10.1039/c1nr11285f
Qian Z, Ma J, Shan X et al (2014) Highly luminescent N-doped carbon quantum dots as an effective multifunctional fluorescence sensing platform. Chemistry 20:2254–2263. https://doi.org/10.1002/chem.201304374
Derfus AM, Chan WCW, Bhatia SN (2004) Probing the cytotoxicity of semiconductor quantum dots. Nano Lett 4:11–18. https://doi.org/10.1021/nl0347334
Liu J, Hu R, Liu J et al (2015) Cytotoxicity assessment of functionalized CdSe, CdTe and InP quantum dots in two human cancer cell models. Mater Sci Eng C 57:222–231. https://doi.org/10.1016/j.msec.2015.07.044
Hu L, Wan J, Zeng G et al (2017) Comprehensive evaluation of the cytotoxicity of CdSe/ZnS quantum dots in: phanerochaete chrysosporium by cellular uptake and oxidative stress. Environ Sci Nano 4:2018–2029. https://doi.org/10.1039/c7en00517b
Nikazar S, Sivasankarapillai VS, Rahdar A et al (2020) Revisiting the cytotoxicity of quantum dots: an in-depth overview. Biophys Rev 12:703–718. https://doi.org/10.1007/s12551-020-00653-0/Published
Xu X, Ray R, Gu Y et al (2004) Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J Am Chem Soc 126:12736–12737. https://doi.org/10.1021/ja040082h
Luo PG, Sahu S, Yang ST et al (2013) Carbon “quantum” dots for optical bioimaging. J Mater Chem B 1:2116–2127. https://doi.org/10.1039/c3tb00018d
Caglayan MO, Mindivan F, Şahin S (2022) Sensor and bioimaging studies based on carbon quantum dots: the green chemistry approach. Crit Rev Anal Chem 52:814–847
Das R, Bandyopadhyay R, Pramanik P (2018) Carbon quantum dots from natural resource: a review. Mater Today Chem 8:96–109
Zhang J, Yu SH (2016) Carbon dots: large-scale synthesis, sensing and bioimaging. Mater Today 19:382–393
Wang L, Li W, Yin L et al (2020) Full-color fluorescent carbon quantum dots. Sci Adv 6(40):eabb6772. https://doi.org/10.1126/sciadv.abb6772
Wang Y, Anilkumar P, Cao L et al (2011) Carbon dots of different composition and surface functionalization: cytotoxicity issues relevant to fluorescence cell imaging. Exp Biol Med 236:1231–1238. https://doi.org/10.1258/ebm.2011.011132
Dong Y, Pang H, Bin YH et al (2013) Carbon-based dots co-doped with nitrogen and sulfur for high quantum yield and excitation-independent emission. Angew Chem Int Ed 52:7800–7804. https://doi.org/10.1002/anie.201301114
Atchudan R, Edison TNJI, Perumal S et al (2019) Green synthesized multiple fluorescent nitrogen-doped carbon quantum dots as an efficient label-free optical nanoprobe for in vivo live-cell imaging. J Photochem Photobiol A Chem 372:99–107. https://doi.org/10.1016/j.jphotochem.2018.12.011
Shen TY, Jia PY, Chen DS, Wang LN (2021) Hydrothermal synthesis of N-doped carbon quantum dots and their application in ion-detection and cell-imaging. Spectrochim Acta A Mol Biomol Spectrosc 248:119282. https://doi.org/10.1016/j.saa.2020.119282
Tadesse A, Hagos M, Ramadevi D et al (2020) Fluorescent-nitrogen-doped carbon quantum dots derived from citrus lemon juice: green synthesis, mercury(II) ion sensing, and live cell imaging. ACS Omega 5:3889–3898. https://doi.org/10.1021/acsomega.9b03175
Tan A, Yang G, Wan X (2021) Ultra-high quantum yield nitrogen-doped carbon quantum dots and their versatile application in fluorescence sensing, bioimaging and anti-counterfeiting. Spectrochim Acta A Mol Biomol Spectrosc 253:119583. https://doi.org/10.1016/j.saa.2021.119583
Bahadar H, Maqbool F, Niaz K, Abdollahi M (2016) Toxicity of nanoparticles and an overview of current experimental models. Iran Biomed J 20:1–11. https://doi.org/10.7508/ibj.2016.01.001
Fard JK, Jafari S, Eghbal MA (2015) A review of molecular mechanisms involved in toxicity of nanoparticles. Adv Pharm Bull 5:447–454
Herzog E, Casey A, Lyng FM et al (2007) A new approach to the toxicity testing of carbon-based nanomaterials-the clonogenic assay. Toxicol Lett 174:49–60. https://doi.org/10.1016/j.toxlet.2007.08.009
Cui D, Tian F, Ozkan CS et al (2005) Effect of single wall carbon nanotubes on human HEK293 cells. Toxicol Lett 155:73–85. https://doi.org/10.1016/j.toxlet.2004.08.015
Pulskamp K, Diabaté S, Krug HF (2007) Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol Lett 168:58–74. https://doi.org/10.1016/j.toxlet.2006.11.001
Wan B, Wang ZX, Lv QY et al (2013) Single-walled carbon nanotubes and graphene oxides induce autophagosome accumulation and lysosome impairment in primarily cultured murine peritoneal macrophages. Toxicol Lett 221:118–127. https://doi.org/10.1016/j.toxlet.2013.06.208
Palomäki J, Karisola P, Pylkkänen L et al (2010) Engineered nanomaterials cause cytotoxicity and activation on mouse antigen presenting cells. Toxicology 267:125–131. https://doi.org/10.1016/j.tox.2009.10.034
Mu Q, Yang L, Davis JC et al (2010) Biocompatibility of polymer grafted core/shell iron/carbon nanoparticles. Biomaterials 31:5083–5090. https://doi.org/10.1016/j.biomaterials.2010.03.020
Yuan X, Zhang X, Sun L et al (2019) Cellular toxicity and immunological effects of carbon-based nanomaterials. Part Fibre Toxicol 16(1):1–27. https://doi.org/10.1186/s12989-019-0299-z
Nurunnabi M, Khatun Z, Huh KM et al (2013) In vivo biodistribution and toxicology of carboxylated graphene quantum dots. ACS Nano 7:6858–6867. https://doi.org/10.1021/nn402043c
Chong Y, Ma Y, Shen H et al (2014) The in vitro and in vivo toxicity of graphene quantum dots. Biomaterials 35:5041–5048. https://doi.org/10.1016/j.biomaterials.2014.03.021
Kim J, Song SH, Jin Y, Park HJ, Yoon H, Jeon S, Cho SW (2016) Multiphoton luminescent graphene quantum dots for in vivo tracking of human adipose-derived stem cells. Nanoscale 8(16):8512–8519. https://doi.org/10.1039/x0xx00000x
Yang ST, Wang X, Wang H et al (2009) Carbon dots as nontoxic and high-performance fluorescence imaging agents. J Phys Chem C 113:18110–18114. https://doi.org/10.1021/jp9085969
Ray SC, Saha A, Jana NR, Sarkar R (2009) Fluorescent carbon nanoparticles: synthesis, characterization, and bioimaging application. J Phys Chem C 113:18546–18551. https://doi.org/10.1021/jp905912n
Niu WJ, Li Y, Zhu RH et al (2015) Ethylenediamine-assisted hydrothermal synthesis of nitrogen-doped carbon quantum dots as fluorescent probes for sensitive biosensing and bioimaging. Sensors Actuators B Chem 218:229–236. https://doi.org/10.1016/j.snb.2015.05.006
Arul V, Edison TNJI, Lee YR, Sethuraman MG (2017) Biological and catalytic applications of green synthesized fluorescent N-doped carbon dots using Hylocereus undatus. J Photochem Photobiol B Biol 168:142–148. https://doi.org/10.1016/j.jphotobiol.2017.02.007
Chousidis I, Stalikas CD, Leonardos ID (2020) Induced toxicity in early-life stage zebrafish (Danio rerio) and its behavioral analysis after exposure to non-doped, nitrogen-doped and nitrogen, sulfur-co doped carbon quantum dots. Environ Toxicol Pharmacol 79:103426. https://doi.org/10.1016/j.etap.2020.103426
Singh V, Kashyap S, Yadav U et al (2019) Nitrogen doped carbon quantum dots demonstrate no toxicity under: in vitro conditions in a cervical cell line and in vivo in Swiss albino mice. Toxicol Res (Camb) 8:395–406. https://doi.org/10.1039/c8tx00260f
Prakash A, Yadav S, Yadav U et al (2023) Recent advances on nitrogen-doped carbon quantum dots and their applications in bioimaging: a review. Bull Mater Sci 46. https://doi.org/10.1007/s12034-022-02846-7
Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002) Introduction to pathogens. In: Molecular Biology of the Cell, 4th edn. Garland Science, pp 1–10
Anaissie EJ, Bodey GP, Rinaldi MG (1989) Emerging fungal pathogens. Eur J Clin Microbiol Infect Dis 8:323–330. https://doi.org/10.1007/BF01963467
Vouga M, Greub G (2016) Emerging bacterial pathogens: the past and beyond. Clin Microbiol Infect 22:12–21. https://doi.org/10.1016/j.cmi.2015.10.010
Pappas PG, Lionakis MS, Arendrup MC et al (2018) Invasive candidiasis. Nat Rev Dis Primers 4:1–20. https://doi.org/10.1038/nrdp.2018.26
Kwon-chung KJ, Fraser JA, Doering TL et al (2014) Cryptococcus neoformans and Cryptococcus gattii, the Etiologic Agents of Cryptococcosis. Cold Spring Harb Perspect Med 4:a019760
Dellière S, Joannard B, Benderdouche M et al (2022) Emergence of diffi cult-to-treat Tinea corporis caused by Trichophyton mentagrophytes complex isolates, Paris, France. Emerg Infect Dis 28:1–5
Sugui JA, Kwon-Chung KJ, Juvvadi PR et al (2015) Aspergillus fumigatus and related species. Cold Spring Harb Perspect Med 5:1–18. https://doi.org/10.1101/cshperspect.a019786
Hunter PR (2003) Drinking water and diarrhoeal disease due to Escherichia coli. J Water Health 1:65–72. https://doi.org/10.2166/wh.2003.0008
Ondusko DS, Nolt D (2018) Staphylococcus aureus. Pediatr Rev 39:287–298. https://doi.org/10.1542/pir.2017-0224
Bodey GP, Bolivar R, Fainstein V, Jadeja L (1983) Infections caused by Pseudomonas aeruginosa. Rev Infect Dis 5(2):279–313
De Medeiros TV, Manioudakis J, Noun F et al (2019) Microwave-assisted synthesis of carbon dots and their applications. J Mater Chem C 7:7175–7195. https://doi.org/10.1039/c9tc01640f
Tan J, Zou R, Zhang J et al (2016) Large-scale synthesis of N-doped carbon quantum dots and their phosphorescence properties in a polyurethane matrix. Nanoscale 8:4742–4747. https://doi.org/10.1039/c5nr08516k
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63. https://doi.org/10.1039/c6ra17788c
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Das K, Samanta L, Chainy GBN (2000) A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. Indian J Biochem Biophys 37:201–204
Claiborne AL (2018) CRC Handbook of Methods for Oxygen Radical Research. CRC Press Taylor & Francis Group
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Ou SF, Zheng YY, Lee SJ et al (2021) N-doped carbon quantum dots as fluorescent bioimaging agents. Crystals 11:1–12. https://doi.org/10.3390/cryst11070789
Sharma N, Sharma I, Bera MK (2022) Microwave-assisted green synthesis of carbon quantum dots derived from calotropis gigantea as a fluorescent probe for bioimaging. J Fluoresc 32:1039–1049. https://doi.org/10.1007/s10895-022-02923-4
Zhao L, Wang Y, Zhao X et al (2019) Facile synthesis of nitrogen-doped carbon quantum dots with chitosan for fluorescent detection of Fe3+. Polymers (Basel) 11:1–12. https://doi.org/10.3390/polym11111731
Nandiyanto ABD, Oktiani R, Ragadhita R (2019) How to read and interpret ftir spectroscope of organic material. Indones. J Sci Technol 4:97–118. https://doi.org/10.17509/ijost.v4i1.15806
Tadesse A, Ramadevi D, Hagos M et al (2018) Synthesis of nitrogen doped carbon quantum dots/magnetite nanocomposites for efficient removal of methyl blue dye pollutant from contaminated water. RSC Adv 8:8528–8536. https://doi.org/10.1039/c8ra00158h
De B, Voit B, Karak N (2013) Transparent luminescent hyperbranched epoxy/carbon oxide dot nanocomposites with outstanding toughness and ductility. ACS Appl Mater Interfaces 5:10027–10034. https://doi.org/10.1021/am402415g
Rahal A, Kumar A, Singh V et al (2014) Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res Int 2014. https://doi.org/10.1155/2014/761264
Jebakumar Immanuel Edison TN, Atchudan R, Karthik N et al (2020) Facile hydrothermal synthesis of nitrogen rich blue fluorescent carbon dots for cell bio-imaging of Candida albicans. Process Biochem 88:113–119. https://doi.org/10.1016/j.procbio.2019.10.003
Liu BR, Huang YW, Chiang HJ, Lee HJ (2010) Cell-penetrating peptide-functionalized quantum dots for intracellular delivery. J Nanosci Nanotechnol 10:7897–7905. https://doi.org/10.1166/jnn.2010.3012
Barroso MM (2011) Quantum dots in cell biology. J Histochem Cytochem 59:237–251. https://doi.org/10.1369/0022155411398487
Acknowledgements
We acknowledge the Council of Scientific and Industrial Research (CSIR), India, for JRF grant (JRF Grant no: 09/013(0897)/2019-EMR-I) for Aakriti Prakash and Institute of Eminence (IoE), BHU, Varanasi, India (Grant no. R/Dev/D/IoE/Incentive/2021–22/32277), for Preeti Suman Saxena. We also acknowledge DST and SATHI-BHU for providing Confocal Laser Scanning Super Resolution Microscope facility for our research work.
Funding
This research work was funded by two funding agencies who financially supported this work.
Author information
Authors and Affiliations
Contributions
Aakriti Prakash: devised the main concept of the work, carried out the experiment, and prepared the manuscript.
Sujit Yadav: helped to analyze and manage data.
Punit Tiwari: helped in providing the strain of fungi and bacteria.
Anchal Srivastava: guidance.
Ragini Tilak: guidance and her lab was used for procurement of microorganisms.
Preeti Suman Saxena: guidance.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 412 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Prakash, A., Yadav, S., Tiwari, P. et al. Facile synthesis of highly fluorescent nitrogen-doped carbon quantum dots and their role in bioimaging of some pathogenic microorganisms. J Nanopart Res 25, 237 (2023). https://doi.org/10.1007/s11051-023-05893-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-023-05893-1