Abstract
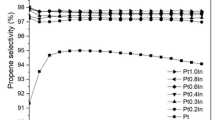
Three γ-Al2O3 supports were prepared by hydrothermal method, and PtSn/Al2O3 catalysts were prepared by sequential impregnation for propane dehydrogenation. The catalyst was characterized by TEM, PXRD, H2-TPR, and other characterization methods. The effects of different morphologies of PtSn/Al2O3 catalysts on the dehydrogenation performance and coking of propane were investigated. The results show that the exposed crystal planes of three morphologies of Al2O3 support are different, which makes the Lewis acid content on the support surface and the interaction of active metals on the support different, thus affecting the catalytic performance of the catalyst and coking carbon.
Graphical Abstract
Different morphologies of γ-Al2O3 expose different crystal planes, change the Lewis acid content on the surface of the support, and regulate the interaction between active metals and support, thus affecting propane dehydrogenation performance and catalyst coking.







Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Natarajan P, Khan HA, Jaleel A et al (2020) The pronounced effect of Sn on RhSn catalysts for propane dehydrogenation. J Catal 392:8–20
Tanabe K, Misono M, Ono Y et al (1989) Acid and base centers: structure and acid-base property. Stud Surf Sci Catal 27–213
Argyle MD, Bartholomew CH (2015) Heterogeneous catalyst deactivation and regeneration: a review. Catalysts 5(1):145–269
Dixit P (2018) Structure-activity relationships in alkane dehydrogenation on gamma-Al2O3: site-dependent reactions [J]. ACS Catal 8(12):11570–11578
Sricharoen C, Jongsomjit B, Panpranot J et al (2020) The key to catalytic stability on sol-gel derived SnOx/SiO2 catalyst and the comparative study of side reaction with K-PtSn/Al2O3 toward propane dehydrogenation-ScienceDirect. Catal Today 375:343–351
Gangwar J, Gupta BK, Tripathi SK et al (2015) Phase-dependent thermal and spectroscopic responses of Al2O3 nanostructures with different morphogenesis. Nanoscale 7(32):13313–13344
Levin I, Brandon D (1998) Metastable alumina polymorphs: crystal structures and transition sequences. J Am Ceram Soc 81(8):1995–2012
Bell TE, González-Carballo JM, Tooze RP et al (2018) High Yield Manufacturing of γ-Al2O3 Nanorods. ACS Sustain Chem Eng 6:88–92
Digne M, Sautet P, Raybaud P et al (2004) Use of DFT to achieve a rational understanding of acid-basic properties of γ-alumina surfaces. J Catal 226(1):54–68
Shi Y, Li X, Rong X et al (2017) Influence of support on the catalytic properties of Pt-Sn-K/θ-Al2O3 for propane dehydrogenation. RSC Adv 7(32):19841–19848
Xu J, Wang Q, Deng F (2019) Metal active sites and their catalytic functions in zeolites: insights from solid-state NMR spectroscopy. Accounts Chem Res 52(8):2179–2189
Yang WY, Ling FX, Wang G et al (2019) Macroporous alumina with three-dimensionally interconnected pore structure: synthesis, characterization and transformation mechanism. J Fuel Chem Technol 47(6):745–750
Dewangan N, Ashok J, Sethia M et al (2019) Cobalt-based catalyst supported on different morphologies of alumina for non-oxidative propane dehydrogenation: effect of metal support interaction and Lewis acidic sites. ChemCatChem 11(19):4923–4934
Shi L, Deng GM, Li WC et al (2015) Al2O3 nanosheets rich in pentacoordinate Al3+ ions stabilize Pt-Sn clusters for propane dehydrogenation. Angew 54(47):13994–13998
Hu M, Kopa D, Likozar B (2020) Kinetics of non-oxidative propane dehydrogenation on Cr2O3 and the nature of catalyst deactivation from first-principles simulations. J Catal 386:126–138
Hu M, Kopa D, Bajec D, Likozar B (2021) Effect of surface oxidation on oxidative propane dehydrogenation over chromia: an ab initio multiscale kinetic study. ACS Catal 11(17):11233–11247
Motagamwal AH, Almallahi R, Wortman J et al (2021) Stable and selective catalysts for propane dehydrogenation operating at thermodynamic limit. Science 373:217–222
Papoian G, Nørskov JK, Hoffmann R (2000) A comparative theoretical study of the hydrogen, methyl, and ethyl chemisorption on the Pt (111) surface. JACS 122(17):4129–4144
Nawaz Z, Tang X, Yao W et al (2010) Parametric characterization and influence of tin on the performance of Pt-Sn/SAPO-34 catalyst for selective propane dehydrogenation to propylene. Ind Eng Chem Res 49(3):1274–1280
Watson GW, Wells R, Willock DJ et al (2016) Density functional theory calculations on the interaction of ethene with the 111 surface of platinum. J Phys Chem B 104(27):6439–6446
Feng J, Liang Z, Li S et al (2015) Propane dehydrogenation over Pt/TiO2-Al2O3 catalysts. ACS Catal 5(1):438–447
Wang HZ, Sun LL, Sui ZJ et al (2018) Coke formation on Pt-Sn/Al2O3 catalyst for propane dehydrogenation. Ind Eng Chem Res 57(26):8647–8654
Chen XY, Huh HS, Lee SW (2007) Hydrothermal synthesis of boehmite (γ-AlOOH) nanoplatelets and nanowires: pH-controlled morphologies. Nanotechnology 18(28):285608
Prakash N, Lee MH, Yoon S et al (2017) Role of acid solvent to prepare highly active PtSn/θ-Al2O3 catalysts in the dehydrogenation of propane to propylene. Catal Today 293:33–41
Clauser AL, Sarfo KO, Giulian R et al (2023) Characterization of the atomic-level structure of γ-alumina and (111) Pt/γ-alumina interfaces. Acta Mater 245:118609
Dehkordi SAH, Golbodaqi M, Mortazavi-Manesh A et al (2023) Dimethyl ether from methanol on mesoporous γ-alumina catalyst prepared from surfactant free highly porous pseudo-boehmite. Mol Catal 538:113004
Mardwita M, Yusmartini ES, Wisudawati N (2020) Effects of cobalt and chromium loadings to the catalytic activities of supported metal catalysts in methane oxidation. Bull Chem React Eng 15(1):213–220
Clauser AL, Giulian R, Mcclure ZD et al (2020) Orientation and morphology of Pt nanoparticles in gamma-alumina processed via ion implantation and thermal annealing. Scripta Mater 188:44–49
Zhu X, Wang T, Xu Z et al (2022) Pt-Sn clusters anchored at Al3+ penta sites as a sinter-resistant and regenerable catalyst for propane dehydrogenation. J Energy Chem 65:293–301
Sharma L, Jiang X, Wu Z et al (2021) Elucidating the origin of selective dehydrogenation of propane on γ-alumina under H2S treatment and co-feed. J Catal 394:142–156
Lieske H, Lietz G, Spindler H et al (1983) Reactions of platinum in oxygen-and hydrogen-treated Ptγ-Al2O3 catalysts: I Temperature-programmed reduction, adsorption, and redispersion of platinum. J Catal 81(1):8–16
Cui ET, Lu GX (2013) Modulating photogenerated electron transfer and hydrogen production rate by controlling surface potential energy on a selectively exposed Pt Facet on Pt/TiO2 for enhancing hydrogen production. J Phys Chem C 117(50):26415–26425
Al-Ansari A, Yadav K, Anderson D et al (2005) Diverse application of unique high-performance water-based-mud technology in the Middle East. SPE/IADC middle east drilling technology conference and exhibition. OnePetro
Li Q, Sui Z, Zhou X et al (2011) Coke formation on Pt-Sn/Al2O3 catalyst in propane dehydrogenation: coke characterization and kinetic study. Top Catal 54:888–896
Iglesias-Juez A, Beale AM, Maaijen K et al (2010) A combined in situ time-resolved UV-Vis, Raman and high-energy resolution x-ray absorption spectroscopy study on the deactivation behavior of Pt and PtSn propane dehydrogenation catalysts under industrial reaction conditions. J Catal 276(2):268–279
Wang X, Cui J, Zhang N et al (2022) Propane dehydrogenation over PtSn/Al2O3 catalysts: influence of urea to Al (NO3)3·9H2O Ratio. Catalysts 12(2):157–169
Khanmohammadi S, Taheri-Nassaj E, Farrokhi-Rad M (2020) Synthesis of meso-porous gamma-alumina membrane: effect of yttria addition on the thermal stability. Surf Interfaces 21:100683
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 91961110, U1908203).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, S., Jiao, J., He, K. et al. PtSn propane dehydrogenation catalyst supported by γ-Al2O3: insight into the supports and active species interaction. J Nanopart Res 25, 238 (2023). https://doi.org/10.1007/s11051-023-05892-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-023-05892-2