Abstract
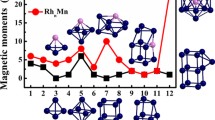
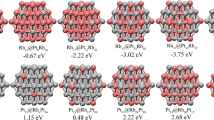
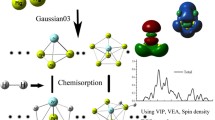
Density functional theory (DFT) studies are performed on the geometric structures, stability, electronic properties of the RhmPdn (m + n = 13) clusters. All the configurations are 13-atomic icosahedral structures, with an atom in the central site. Rh1Pd12 has Ih symmetry and relatively suitable stability and electronic properties among these clusters, with moderate stability and inter-atomic binding interaction as the representative system for the study of NO molecule adsorption and dissociation. NO molecule tends to be end-on adsorbed on Rh1Pd12 with its N atom connecting to metal atoms of the cluster, preferably at the hollow site to the top or bridge site. For the dissociation of NO as a key step in its reduction, the adsorption and dissociation of NO on Rh1Pd12 cluster are overall exothermic reactions from the energies of the free NO molecule and the cluster. Doping Rh in the pure cluster affects little on the adsorption energy and dissociation energy barrier but much on the relative energy (the energy reduction from the free NO molecule and cluster to the final state).









Similar content being viewed by others
Data Availability
The authors do not have permission to share data.
References
Cleveland CL, Landman U (1991) The energetics and structure of nickel clusters: size dependence. J Chem Phys 94:7376–7396
Apai G, Hamilton JF, Stohr J, Thompson A (1979) Extended X-ray—absorption fine structure of small Cu and Ni clusters: binding-energy and bond-length changes with cluster size. Phys Rev Lett 43:165–169
Molayem M, Grigoryan VG, Springborg M (2011) Global minimum structures and magic clusters of CumAgn nanoalloys. J Phys Chem C 115:22148–22162
Sasikala Devi AA (2014) Structural, magnetic and electronic properties of FexCoyIrz (x + y + z = 5, 6) clusters: an ab initio study. Eur Phys J D 68:120
Baletto F, Ferrando R (2005) Structural properties of nanoclusters: energetic, thermodynamic, and kinetic effects. Rev Mod Phys 77:371–423
Howalt JG, Bligaard T, Rossmeisl J, Vegge T (2013) DFT based study of transition metal nano-clusters for electrochemical NH3 production. Phys Chem Chem Phys 15:7785–7795
Yudanov IV, Genest A, Schauermann S, Freund HJ, Rosch N (2012) Size dependence of the adsorption energy of CO on metal nanoparticles: a DFT search for the minimum value. Nano Lett 12:2134–2139
Li S, Hennigan JM, Dixon DA, Peterson KA (2009) Accurate thermochemistry for transition metal oxide clusters. J Phys Chem A 113:7861–7877
Montejo-Alvaro F, Oliva J, Zarate A, Herrera-Trejo M, Hdz-García HM, Mtz-Enriquez AI (2019) Icosahedral transition metal clusters (M13, M = Fe, Ni, and Cu) adsorbed on graphene quantum dots, a DFT study. Phys E: Low-Dimens Syst Nanostructures 110:52–58
Ji M, Gu X, Li X, Gong X, Li J, Wang LS (2005) Experimental and theoretical investigation of the electronic and geometrical structures of the Au32 cluster. Angew Chem Int Ed Eng 44:7119–7123
Rexer EF, Jellinek J, Krissinel EB, Parks EK, Riley SJ (2002) Theoretical and experimental studies of the structures of 12-, 13-, and 14-atom bimetallic nickel/aluminum clusters. J Chem Phys 117:82–94
Zhou J-M, Shi W, Li H-M, Li H, Cheng P (2013) Experimental Studies and mechanism analysis of high-sensitivity luminescent sensing of pollutional small molecules and ions in Ln4O4 cluster based microporous metal–organic frameworks. J Phys Chem C 118:416–426
Ferrando R, Jellinek J, Johnston RL (2008) Nanoalloys: from theory to applications of alloy clusters and nanoparticles. Chem Rev 108:845–910
Yan L, Wu S-Y, Yang Y, Hu J-G, Wei Z-T, Zhong S-Y (2022) Density functional theory study of BiRun (n = 3–20) clusters: structural, electronic and adsorptive properties for hazardous gases. Comput Theor Chem 1209:113623
Zhong S-Y, Wu S-Y, Guo J-X, Shen G-Q, Li X-Y, Xu K-L (2022) Theoretical investigations on structural, electronic, adsorptive and catalytic properties of BiPdn (n = 2 - 20) bimetallic nanoclusters. J Non-Cryst Solids 594:121801
Zhang CJ, Hu P (2001) CO oxidation on Pd(100) and Pd(111): a comparative study of reaction pathways and reactivity at low and medium coverages. J Am Chem Soc 123:1166–1172
Kunz S, Schweinberger FF, Habibpour V, Röttgen M, Harding C, Arenz M, Heiz U (2009) Temperature dependent CO oxidation mechanisms on size-selected clusters. J Phys Chem C 114:1651–1654
Worz AS, Judai K, Abbet S, Heiz U (2003) Cluster size-dependent mechanisms of the CO + NO reaction on small Pdn (n < or = 30) clusters on oxide surfaces. J Am Chem Soc 125:7964–7970
Lee S, Lee B, Mehmood F, Seifert S, Libera JA, Elam JW, Greeley J, Zapol P, Curtiss LA, Pellin MJ, Stair PC, Winans RE, Vajda S (2010) Oxidative decomposition of methanol on subnanometer palladium clusters: the effect of catalyst size and support composition. J Phys Chem C 114:10342–10348
Kaneeda M, Iizuka H, Hiratsuka T, Shinotsuka N, Kitahara Y, Arai M (2010) Preparation and screening of various multi-component catalysts for NOx conversion under lean-burn conditions: an active and heat-resistant RhPt–NaMn–Ce/Al2O3 catalyst. Chem Eng J 160:93–98
Wang J, Hao W, Ma L-J, Jia J, Wu H-S (2019) The effect of interstitial boron on the mechanisms of acetylene hydrogenation catalyzed by Pd6: a DFT study. Comput Theor Chem 1170:112636
Lang SM, Fleischer I, Bernhardt TM, Barnett RN, Landman U (2015) Low-temperature CO oxidation catalyzed by free palladium clusters: similarities and differences to Pd surfaces and supported particles. ACS Catal 5:2275–2289
Li SJ, Zhou X, Tian WQ (2012) Theoretical investigations on decomposition of HCOOH catalyzed by Pd7 cluster. J Phys Chem A 116:11745–11752
Zhou C, Yao S, Wu J, Forrey RC, Chen L, Tachibana A, Cheng H (2008) Hydrogen dissociative chemisorption and desorption on saturated subnano palladium clusters (Pdn, n = 2-9). Phys Chem Chem Phys 10:5445–5451
Jeon J, Yu BD (2015) Dynamics of Pd monomers and dimers adsorbed on the (001) surfaces of strongly correlated nickel oxides. Curr Appl Phys 15:98–102
Omar S, Palomar J, Gómez-Sainero LM, Álvarez-Montero MA, Martin-Martinez M, Rodriguez JJ (2011) Density functional theory analysis of dichloromethane and hydrogen interaction with Pd clusters: first step to simulate catalytic hydrodechlorination. J Phys Chem C 115:14180–14192
Yudanov IV, Genest A, Rösch N (2011) DFT Studies of palladium model catalysts: structure and size effects. J Clust Sci 22:433–448
Ozensoy E, Wayne Goodman D (2004) Vibrational spectroscopic studies on CO adsorption, NO adsorption CO + NO reaction on Pd model catalysts. Phys Chem Chem Phys 6:3765–3778
Ni M, Zeng Z (2009) Density functional study of hydrogen adsorption and dissociation on small Pdn (n = 1–7) clusters. J Mol Struct Theochem 910:14–19
Liu X, Tian D, Meng C (2015) DFT study on the adsorption and dissociation of H2 on Pdn (n = 4, 6, 13, 19, 55) clusters. J Mol Struct 1080:105–110
Dutta A, Mondal P (2018) Density functional studies on structural, electronic and magnetic properties of Rhn (n = 9–20) clusters and O–H bond of methanol activation by pure and ruthenium-doped rhodium clusters. Theor Chem Accounts 138:7
Sun H, Ren Y, Luo Y-H, Wang G (2001) Geometry, electronic structure, and magnetism of clusters. Phys B Condens Matter 293:260–267
Reddy BV, Nayak SK, Khanna SN, Rao BK, Jena P (1999) Electronic structure and magnetism of Rhn (n = 2–13)clusters. Phys Rev B 59:5214–5222
Hang TD, Hung HM, Thiem LN, Nguyen HMT (2015) Electronic structure and thermochemical properties of neutral and anionic rhodium clusters Rhn, n = 2–13. Evolution of structures and stabilities of binary clusters RhmM (M=Fe, Co, Ni; m = 1–6). Comput Theor Chem 1068:30–41
Luo C, Zhou C, Wu J, Dhilip Kumar TJ, Balakrishnan N, Forrey RC, Cheng H (2007) First principles study of small palladium cluster growth and isomerization. Int J Quantum Chem 107:1632–1641
El-Bayyari Z, Oymak H, Kökten H (2011) On the structural and energetic features of small metal clusters: Nin, Cun, Pdn, Ptn, and Pbn; n = 3–13. Int J Mod Phys C 15:917–930
Liu Z, Hao J, Fu L, Zhu T (2003) Study of Ag/La0.6Ce0.4CoO3 catalysts for direct decomposition and reduction of nitrogen oxides with propene in the presence of oxygen. Appl Catal B Environ 44:355–370
Liu Z, Li J, Woo SI (2012) Recent advances in the selective catalytic reduction of NOx by hydrogen in the presence of oxygen. Energy Environ Sci 5:8799–8814
Daté M, Okuyama H, Takagi N, Nishijima M, Aruga T (1996) Interaction of NO with CO on Pd(100): ordered coadsorption structures and explosive reaction. Surf Sci 350:79–90
Rainer DR, Vesecky SM, Koranne M, Oh WS, Goodman DW (1997) The CO+NO reaction over Pd: a combined study using single-crystal, planar-model-supported, and high-surface-area Pd/Al2O3 catalysts. J Catal 167:234–241
Thirunavukkarasu K, Thirumoorthy K, Libuda J, Gopinath CS (2005) A molecular beam study of the NO + CO reaction on Pd(111) surfaces. J Phys Chem B 109:13272–13282
Viñes F, Desikusumastuti A, Staudt T, Görling A, Libuda J, Neyman KM (2008) A combined density-functional and IRAS study on the interaction of NO with Pd nanoparticles: identifying new adsorption sites with novel properties. J Phys Chem C 112:16539–16549
de Wolf CA, Nieuwenhuys BE (2000) The NO–H2 reaction over Pd(111). Surf Sci 469:196–203
Ma Y, Matsushima T (2005) Surface-nitrogen removal in a steady-state NO + H2 reaction on Pd(110). J Phys Chem B 109:1256–1261
Huai LY, He CZ, Wang H, Wen H, Yi WC, Liu JY (2015) NO dissociation and reduction by H 2 on Pd(1 1 1): a first-principles study. J Catal 322:73–83
Grybos R, Benco L, Bucko T, Hafner J (2009) Molecular adsorption and metal-support interaction for transition-metal clusters in zeolites: NO adsorption on Pd(n) (n = 1-6) clusters in mordenite. J Chem Phys 130:104503
Weiss BM, Iglesia E (2010) Mechanism and site requirements for NO oxidation on Pd catalysts. J Catal 272:74–81
Gao Y, Zhang LM, Kong CC, Yang ZM, Chen YM (2016) NO adsorption and dissociation on palladium clusters: the importance of charged state and metal doping. Chem Phys Lett 658:7–11
Liu X, Tian D, Ren S, Meng C (2015) Structure sensitivity of NO adsorption–dissociation on Pdn (n = 8, 13, 19, 25) clusters. J Phys Chem C 119:12941–12948
Lacaze-Dufaure C, Roques J, Mijoule C, Sicilia E, Russo N, Alexiev V, Mineva T (2011) A DFT study of the NO adsorption on Pdn (n = 1–4) clusters. J Mol Catal A Chem 341:28–34
Yin Z, Li C, Su Y, Liu Y, Wang Y, Chen G (2012) Investigation of reaction mechanisms of NO with CO on Pd1/MgO and Pd4/MgO catalysts. Chem Phys 395:108–114
Ahmed F, Nagumo R, Miura R, Suzuki A, Tsuboi H, Hatakeyama N, Takaba H, Miyamoto A (2011) CO oxidation and NO reduction on a MgO(100) supported Pd cluster: a quantum chemical molecular dynamics study. J Phys Chem C 115:24123–24132
Inderwildi OR, Jenkins SJ, King DA (2007) When adding an unreactive metal enhances catalytic activity: NOx decomposition over silver–rhodium bimetallic surfaces. Surf Sci 601:L103–L108
González S, Sousa C, Illas F (2006) Promoter and poisoning effects on NO-catalyzed dissociation on bimetallic RhCu(111) surfaces. J Catal 239:431–440
Gonzalez S, Sousa C, Illas F (2005) Theoretical study of CO and NO chemisorption on RhCu(111) surfaces. J Phys Chem B 109:4654–4661
González S, Loffreda D, Sautet P, Illas F (2007) Theoretical study of NO dissociation on stepped Rh(221) and RhCu(221) surfaces. J Phys Chem C 111:11376–11383
Harada M, Asakura K, Toshima N (1993) Structural analysis of polymer-protected Pd/Rh bimetallic clusters by using EXAFS spectroscopy. Jpn J Appl Phys 32:451
Ugalde M, Chavira E, Ochoa-Lara MT, Figueroa IA, Quintanar C, Tejeda A (2013) Synthesis by microwaves of bimetallic nano-rhodium-palladium. J Nanotechnol 2013:1–9
Delley B (2000) From molecules to solids with the DMol3 approach. J Chem Phys 113:7756–7764
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Delley B (1990) An all-electron numerical method for solving the local density functional for polyatomic molecules. J Chem Phys 92:508–517
Tkatchenko A, Scheffler M (2009) Accurate molecular van der Waals interactions from ground-state electron density and free-atom reference data. Phys Rev Lett 102:073005
Lopez N, Norskov JK (2002) Catalytic CO oxidation by a gold nanoparticle: a density functional study. J Am Chem Soc 124:11262–11263
Baraiya BA, Mankad V, Jha PK (2020) Uncovering the structural, electronic and vibrational properties of atomically precise PdmCun clusters and their interaction with CO2 molecule. Spectrochim Acta A Mol Biomol Spectrosc 229:117912
Cantera-López H, Montejano-Carrizales JM, Aguilera-Granja F, Morán-López JL (2010) Theoretical study of bimetallic magnetic nanostructures: ConPdN-n, n = 0,1,...N, N = 3,5,7,13. Eur Phys J D 57:61–69
Jinlong Y, Toigo F, Kelin W, Manhong Z (1994) Anomalous symmetry dependence of Rh13 magnetism. Phys Rev B Condens Matter 50:7173–7176
Qiu G, Wang M, Wang G, Diao X, Zhao D, Du Z, Li Y (2008) Structure and electronic properties of Pd clusters and their interactions with single S atom studied by density-functional theory. J Mol Struct Theochem 861:131–136
Alonso JA, Girifalco LA (1979) Electronegativity scale for metals. Phys Rev B 19:3889–3895
Honkala K, Pirilä P, Laasonen K (2001) CO and NO adsorption and co-adsorption on the Pd(111) surface. Surf Sci 489:72–82
Jigato MP, Somasundram K, Termath V, Handy NC, King DA (1997) Vibrational frequencies for NO chemisorbed on different sites: DFT calculations on Pd clusters. Surf Sci 380:83–90
Seyferth D (1978) Infrared and raman spectra of inorganic and coordination compounds. J Organomet Chem 156:C47–C48
Su B-F, Fu H-Q, Yang H-Q, Hu C-W (2015) Catalytic reduction of NO by CO on Rh4+ clusters: a density functional theory study. Catal Sci Technol 5:3203–3215
Chang CC, Ho JJ (2014) Catalytic enhancement in dissociation of nitric oxide over rhodium and nickel small-size clusters: a DFT study. Phys Chem Chem Phys 16:5393–5398
Acknowledgements
The authors are grateful for Prof. Kai-Lai Xu of Key Laboratory of Green Chemistry & Technology of Ministry of Education at Sichuan University for her kind help in calculations with MS software.
Funding
Natural Science Foundation of Jiangxi (No. 20212BAB201021) and Chengdu Innovation and Technology Project Granted (No. 2021-YF05–02413-GX).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, XX., Wu, SY., Guo, TH. et al. Density functional theory studies on the structures and NO molecule adsorption and dissociation of RhmPdn (m + n = 13) clusters. J Nanopart Res 25, 236 (2023). https://doi.org/10.1007/s11051-023-05884-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-023-05884-2