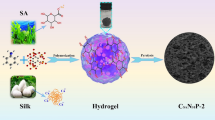
Abstract
The cost-effective and green preparation of electrocatalysts that are highly effective for both the cathode (reduction of oxygen) and anode (methanal oxidation) reactions is crucial for boosting the development of Direct Methanol fuel cells (DMFCs). This article describes a novel designed nitrogen and phosphorous co-doped honeycomb-like electrocatalyst (CAMN6P-3), which is derived from a hydrogel network formed with silk protein, polyaniline, and polyacrylamide. Notably, the CAMN6P-3 material has a surprisingly high nitrogen content (6.82%) and a high specific surface area (2494.55 m2 g−1). A high onset potential (1.11 V) was observed for the CAMN6P-3 catalyst, as well as a high limiting current density (5.4 mA.cm−2) during ORR. Furthermore, Pt/CAMN6P-3 demonstrates considerable catalytic activity as well as electrochemical stability for MOR as a catalyst carrier for Pt nanoparticles (Pt NPs). After long-term stability tests, the current density in the MOR was equivalent to a factor of twenty times that of the catalyst made from Pt/C. This superior performance can be attributed to the special nanostructure, where the honeycomb nanostructure not only provides an efficient channel for electron transfer and exposes more active sites, but also facilitates a high degree of dispersion of the Pt nanoparticles. From the perspective of sustainable development, combined with low-cost materials and the green preparation process, this work has a certain reference value for the development of highly efficient catalysts in the field of clean fuel cells.









Similar content being viewed by others
References
Wang Y, Luo S, Pan W, Leung DYC (2021) Microfluidic fuel cells with different types of fuels: A prospective review. Renew Sust Energ Rev 141:110806
Stamenkovic VR, Strmcnik D, Lopes PP (2016) Energy and fuels from electrochemical interfaces. Nat Mater 16:57–69
Neaţu S, Neaţu F, Chirica IM (2021) Recent progress in electrocatalysts and electrodesfor portable fuel cells. Mater Chem A 9:17065–17128
Pan ZF, An I, Zhao TS (2018) Advances and challenges in alkaline anion exchange membrane fuel cells. Prog Energy Combust Sci 66:141–175
Ioroi T, Siroma Z, Yamazaki SI (2018) Electrocatalysts for PEM Fuel Cells. Adv Energy Mater 9:1801284
Liu X, Zhai S, Zhang X (2021) Efficient and durable composite membranes based on polydopamine-mediated sulfonated graphene oxide for direct methanol fuel cells. J Mater Chem A 9:24044
Xiao F, Wang YC, Wu ZP (2021) Recent Advances in Electrocatalysts for Proton Exchange Membrane Fuel Cells and Alkaline Membrane Fuel Cells. Adv Mater 33:2006292
Zuo Y, Sheng W, Tao W (2022) Direct methanol fuel cells system-A review of dual-role electrocatalysts for oxygen reduction and methanol oxidation. J Mater Sci Technol 114:29–41
Li S, Tang X, Jia H (2020) Nanoporous high-entropy alloys with low Pt loadings for high performance electrochemical oxygen reduction. J Catal 383:164–171
Shao MH, Chang QW, Dodelet JP (2016) Recent advances in electrocatalysts for oxygen reduction reaction. Chem Rev 116:3594–3657
Huang L, Zaman S, Tian X (2021) Advanced Platinum-Based Oxygen Reduction Electrocatalysts for Fuel Cells. Acc Chem Res 54:311–322
Wang P, Shao Q, Huang X (2018) Updating Pt-Based Electrocatalysts for Practical Fuel Cells. Joule 2:2514–2516
Zhang C, Li J, Li C (2021) Surface and interface engineering of hollow carbon sphere-based electrocatalysts for the oxygen reduction reaction. J Mater Chem A 9:25706
Serov A, Kwak C (2009) Review of non-platinum anode catalysts for DMFC and PEMFC application. Appl Catal B Environ 90:313–320
Zhao L, Sui XL, Li JZ (2018) Supramolecular assembly promoted synthesis of three-dimensional nitrogen doped graphene frameworks as efficient electrocatalyst for oxygen reduction reaction and methanol electrooxidation. Appl Catal B Environ 231:224–233
Yang Q, Xiao Z, Kong D (2019) New insight to the role of edges and heteroatoms in nanocarbons for oxygen reduction reaction. Nano Energy 66:104096
Razmjooei F, Singh KP, Song MY (2014) Enhanced electrocatalytic activity due to additional phosphorous doping in nitrogen and sulfur-doped graphene: A comprehensive study. Carbon 78:257–267
Yang L, Zeng X, Wang W (2018) Recent Progress in MOF-Derived, Heteroatom-Doped Porous Carbons as Highly Efficient Electrocatalysts for Oxygen Reduction Reaction in Fuel Cells. Adv Funct Mater 28:1704537
Li JC, Hou PX, Liu C (2017) Heteroatom-Doped Carbon Nanotube and Graphene-Based Electrocatalysts for Oxygen Reduction Reaction. Small 13:1702002
Marbaniang P, Ingavale S, Karuppanan P (2021) Rationale approach of nitrogen doping at defect sites of multiwalled carbon nanotubes: A strategy for oxygen reduction electrocatalysis. Int J Hydrogen Energy 46:10268–10280
Wang W, Zhao X, Shi H (2020) Shape inducer-free Polygonal Angle Platinum Nanoparticles in Graphene Oxide as Oxygen Reduction Catalyst derived from Gamma Irradiation. J Colloid Interf Sci 575:1–15
Liu H, Cheng J, Lu Z (2019) Significant enhancement of the oxygen reduction activity of self-heteroatom doped coal derived carbon through oxidative pretreatment. Electrochim Acta 12:22–30
Wang L, Liang K, Deng L (2019) Protein hydrogel networks: A unique approach to heteroatom self-doped hierarchically porous carbon structures as an efficient ORR electrocatalyst in both basic and acidic conditions. Appl Catal B: Environ 246:89–99
Li D, Yang D, Yang X (2016) Double-helix structure in carrageenan-metal hydrogels: a general approach to porous metal sulfides/carbon aerogels with excellent sodium-ion storage. Angew Chem Int Ed 55:15925–15928
Wang C, Li Z, Wang L (2019) Facile synthesis of 3D Fe/N codoped mesoporous graphene as efficient bi-functional oxygen electrocatalysts for rechargeable Zn-air batteries. ACS Sustain Chem Eng 7:7
Chen M, Jiang Y, Mei P (2019) Polyacrylamide Microspheres-Derived Fe3C@N-doped Carbon Nanospheres as Efficient Catalyst for Oxygen Reduction Rectiaon. Polymers 11:767
Wang C, Xie N-H, Zhang Y (2019) Silk-Derived Highly Active Oxygen Electrocatalysts for Flexible and Rechargeable Zn-Air Batteries. Chem Mater 31:1023–1029
Tian P, Wang Y, Li W (2020) A salt induced gelatin crosslinking strategy to prepare Fe-N doped aligned porous carbon for efficient oxygen reduction reaction catalysts and high-performance supercapacitors. J Catal 382:109–120
Wang H, Dou S, Wang S (2017) Synthesis of electrocatalytically functional carbon honeycombs through cooking with molecule precursors. In J Hydrogen Energ 42:6472–6481
Zhao Z, Long Y, Chen Y (2022) Phosphorus doped carbon nitride with rich nitrogen vacancy to enhance the electrocatalytic activity for nitrogen reduction reaction. Chem Eng J 430:132682
Jiang H, Gu J, Zheng X (2019) Defect-rich and ultrathin N doped carbon nanosheets as advanced trifunctional metal-free electrocatalysts for the ORR, OER and HER. Energy Environ Sci 12:322–333
Wu M, Wang Y, Wei Z (2018) Ternary doped porous carbon nanofibers with excellent ORR and OER performance for zinc-air batteries. J Mater Chem A 6:10918–10925
Wang H, Zhang W, Bai P (2020) Ultrasound-assisted transformation from waste biomass to efficient carbon-based metal-free pH-universal oxygen reduction reaction electrocatalysts. Ultrason Sonochem 65:05048
Xing Z, Jin R, Chen X (2021) Self-templating construction of N, P-co-doped carbon nanosheets for efficient eletreocatalytic oxygen reduction reaction. Chem Eng J 410:128015
Liu L, Zeng G, Chen J (2018) N-doped Porous Carbon Nanosheets as pH- universal ORR Electrocatalyst in Various Fuel Cell Devices. Nano Energy 8:393–402
Macchi S, Watanabe F, Viswanathan T (2021) Characterization and Electrocatalytic Performance of Molasses Derived Co-Doped (P, N) and Tri-Doped (Si, P, N) Carbon for the ORR. Electrochem 2:311–322
Lei W, Deng Y-P, Li G (2018) Two-Dimensional Phosphorus-Doped Carbon Nanosheets with Tunable Porosity for Oxygen Reactions in Zinc-Air Batteries. ACS Catal 8:2464–2472
Chai GL, Qiu K, Qiao M (2017) Active sites engineering leads to exceptional ORR and OER bifunctionality in P, N Co-doped graphene frameworks. Energ Environ Sci 10:1186–1195
Liu Q, Zhou Y, Chen S (2015) Cellulose-derived nitrogen and phosphorus dual-doped carbon as high performance oxygen reduction catalyst in microbial fuel cell. J Power Sources 273:1189–1193
Shi C, Maimaitiyiming X (2021) Three dimensional nitrogen, phosphorus and sulfur doped porous graphene as efficient bifunctional electrocatalysts for direct methanol fuel cell. In J Hydrogen Energ 46:10247–10258
Siahkalroudi ZM, Aghabarari B, Vaezi M (2021) Effect of secondary heteroatom (S, P) in N-doped reduced graphene oxide catalysts to oxygen reduction reaction. Mol Catal 502:111372
Mao X, Cao Z, Chen S (2019) Facile synthesis of N, P-doped hierarchical porous carbon framework catalysts based on gelatin/phytic acid supermolecules for electrocatalytic oxygen reduction. J Hydrogen Energy 44:5890–5898
Jian G, He C, Liu J (2018) Polymerizable ionic liquid as precursor for N, P co-doped carbon toward oxygen reduction reaction. Catal Sci Technol 8:1142–1150
Alonso-Lemus IL, Figueroa-Torres MZ, Lardizabal-Gutíerrez D (2019) Converting chicken manure into highly active N-P co-doped metal-free biocarbon electrocatalysts:effect of chemical treatment on their catalytic activity for the ORR. Sustain Energ Sustain Energ Fuels 3:1307–1316
Quílez-Bermejo J, Morall E, Cazorla-Amor D (2020) Metal-free heteroatom-doped carbon-based catalysts for ORR: A critical assessment about the role of heteroatoms. Carbon 165:434–454
Zhang J, Wang R, Hua X (2020) MOF-derived Co embedded into N-doped nanotube decorated mesoporous carbon as a robust support of Pt catalyst for methanol electrooxidation-ScienceDirect. Appl Surf Sci 533:147319
Yang C, Jiang Q, Li W (2019) Ultrafine Pt Nanoparticle-Decorated 3D Hybrid Architectures Built from Reduced Graphene Oxide and MXene Nanosheets for Methanol Oxidation. Chem Mater 31:9277–9287
An M, Du L, Du C (2018) Pt nanoparticles supported by sulfur and phosphorus co-doped graphene as highly active catalyst for acidic methanol electrooxidation. Electrochim Acta 285:202–213
Pongpichayakul N, Themsirimongkon S, Maturost S (2021) Cerium oxide-modified surfaces of several carbons as supports for a platinum-based anode electrode for methanol electro-oxidation. In J Hydrogen Energ 46:2905–2916
Zhong J, Wu L, Lan J (2020) Worm-like Pt nanoparticles anchored on graphene with S, N co-doping and Fe3O4 functionalization for boosting the electrooxidation of methanol. In J Hydrogen Energ 45:22929–22937
Mu X, Xu Z, Ma Y (2017) Graphene-carbon nanofiber hybrid supported Pt nanoparticles with enhanced catalytic performance for methanol oxidation and oxygen reduction. Electrochim Acta 253:171–177
Guo Q, Lu X, Fei G (2022) Nitrogen-Doped Graphene Aerogel Microspheres Used as Electrocatalyst Supports for Methanol Oxidation. Ind Eng Chem Res 61:1398–1407
Gorle DB, Kulandainathan MA (2017) One-pot synthesis of highly efficient graphene based three-dimensional hybrids as catalyst supporting materials for electro-oxidation of liquid fuels. J Mater Chem A. 5:15273–15286
Su Z, Li C, Cheng Y (2018) Enhanced electrocatalytic performance of platinum nanoparticles on thiolated polyaniline multiwalled carbon nanotubes for methanol oxidation. RSC Adv 8:33742
Liang X, Dong F, Tang Z (2022) The Pt/g-C3N4-CNS catalyst via in situ synthesis process with excellent performance for methanol electrocatalytic oxidation reaction. New J Chem 46:3121
Choi SM, Min HS, Kim HJ (2011) Synthesis of surface-functionalized graphene nanosheets with high Pt-loadings and their applications to methanol electrooxidation. Carbon 49:904–909
Ali A, Shen PK (2019) Recent advances in graphene-based platinum and palladium electrocatalysts for the methanol oxidation reaction. J Mater Chem A 7:22189–22217
An GH, Gi JH, Jin AH (2018) Platinum nanoparticles on nitrogen-doped carbon and nickel composites surfaces: A high electrical conductivity for methanol oxidation reaction. J Alloys Compd 763:250–256
Chen D, He Z, Pei SE (2019) Pd nanoparticles supported on N and P dual-doped graphene as an excellent composite catalyst for methanol electro-oxidation. J Alloys Compd 785:781–788
Acknowledgements
The authors appreciate the support from Key Research and Development Projects of Xinjiang Uygur Autonomous Region:«Research on Key Technologies for Pre-Treatment of Agroforestry Waste»(2022B02013-1) and the Opening Foundation of Key Laboratory of Xinjiang Uygur Autonomous Region (2020D04001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yisilamu, Z., Zhao, X., Maimaitiyiming, X. et al. Hydrogel derived N, P co-doped 3D honeycomb-like nano porous carbon: CAMN6P-3 and its electrochemical properties. J Nanopart Res 25, 213 (2023). https://doi.org/10.1007/s11051-023-05859-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-023-05859-3