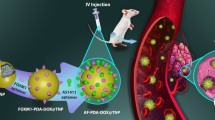
Abstract
Paclitaxel (PTX), a chemo-drug widely used in cancer chemotherapy for a variety of tumors, has been still faced with several problems in therapeutic applications due to its poor water solubility, low content in natural sources, expensive multistep processes of synthesis and side effects. In this study, a nanogel system based on the conjugation of Heparin and Pluronic F127 via disulphide bridges was developed for PTX delivery in a redox responsive way to over these mentioned drawbacks. The obtained Hep-F127 system were proved and characterized through H-NMR, zeta potential, DLS, TEM and FT-IR methods. TEM result showed that the nanogel Hep-F127 was spherical, non-agglomerated and relatively uniform with an average particle diameter of 80 nm. PTX was effectively encapsulated into the nanogel thanks to poly(propylene oxide) (PPO) units of F127 molecules with DLE and DLC values of about 92% and 18%, respectively. Meanwhile, the nanogel was stable in physiological condition but broken under reducing condition, leading to a well-controlled release in physiological condition and a fast release in simulating tumor-microenvironmental condition. This contributed to reducing side effects and increasing the effectiveness of treatment of PTX. In addition, cytotoxicity of Hep-F127 and PTX@Hep-F127 was in vitro tested on the L929 cell line. This study suggested that the nanogel Hep-F127 would to be a promising carrier for enhancing the solubility of PTX and release PTX in a redox-responsive way in cancer treatment.








Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Lim PT, Goh BH, Lee W-L (2022) Taxol: Mechanisms of action against cancer, an update with current research. Paclitaxel, Elsevier, 47–71 https://doi.org/10.1016/B978-0-323-90951-8.00007-2
Zhu L, Chen L (2019) Progress in research on paclitaxel and tumor immunotherapy. Cell Mol Biol Lett 24:1–11. https://doi.org/10.1186/s11658-0190164-y
Lu J, Xu F, Rao C, Shen C, Jin J, Zhu Z, Wang C, Li Q (2022) Mechanism of action of paclitaxel for treating glioblastoma based on single-cell RNA sequencing data and network pharmacology. Front Pharmacol 13. https://doi.org/10.3389/fphar.2022.1076958
Ma P, Mumper RJ (2013) Paclitaxel nano-delivery systems: a comprehensive review. J Nanomedicine Nanotechnol 4:1000164. https://doi.org/10.4172/2157-7439.1000164
Begines B, Ortiz T, Pérez-Aranda M, Martínez G, Merinero M, Argüelles-Arias F, Alcudia A (2020) Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 10:1403. https://doi.org/10.3390/nano10071403
Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R (2021) Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov 20:101–124. https://doi.org/10.1038/s41573-020-0090-8
Le Trang NT, Nguyen NH, Hoang MC, Khoa Nguyen C, Hai Nguyen D, Tran DL (2022) Preparation of liposomal nanocarrier by extruder to enhance tumor accumulation of paclitaxel. J Bioact Compat Polym 37:3–16. https://doi.org/10.1177/08839115211053926
Yin Y, Hu B, Yuan X, Cai L, Gao H, Yang Q (2020) Nanogel: A versatile nano-delivery system for biomedical applications. Pharmaceutics 12:290. https://doi.org/10.3390/pharmaceutics12030290
Suhail M, Rosenholm JM, Minhas MU, Badshah SF, Naeem A, Khan KU, Fahad M (2019) Nanogels as drug-delivery systems: A comprehensive overview. Ther Deliv 10:697–717. https://doi.org/10.4155/tde-2019-0010
Nguyen DHT, Nguyen DYP, Pham LPT, Vo TNN, Nguyen DH, Park KD (2018) Preparation and characterization of redox-sensitive pluronic F127-based nanogel as effective nanocarrier for drug delivery. Int Conf Dev Biomed Eng Vietnam, Springer, pp. 189-192.https://doi.org/10.1007/978-981-13-5859-332
Lin H-H, Robertson KL, Don SSL, Taylor SR, Farkas ME (2020) Chemical modulation of circadian rhythms and assessment of cellular behavior via indirubin and derivatives. Methods Enzymol, Elsevier, pp. 115–140. https://doi.org/10.1016/bs.mie.2020.04.011
Lavogina D, Lust H, Tahk M-J, Laasfeld T, Vellama H, Nasirova N, Vardja M, Eskla K-L, Salumets A, Rinken A (2022) Revisiting the resazurin-based sensing of cellular viability: widening the application horizon. Biosensors 12:196. https://doi.org/10.3390/bios12040196
Note A (2015) 33 Live/dead staining with FDA and PI. ibidi GmbH 2015. Accessed 10 Sep 2022
Liu Y, Fu S, Lin L, Cao Y, Xie X, Yu H, Chen M, Li H (2017) Redox-sensitive Pluronic F127-tocopherol micelles: synthesis, characterization, and cytotoxicity evaluation. Int J Nanomed 12:2635. https://doi.org/10.2147/IJN.S122746
Yan B, Zhou H, Lai J, Wang Z, Luo C, Liu H, Jin X, Ma A, Chen W (2019) Pluronic F127 gels fabricated by thiol–ene click chemistry: Preparation, gelation dynamics, swelling behaviors and mechanical properties. Polym Bull 76:6049–6061. https://doi.org/10.1007/s00289-019-02696-0
Gujar V, Pathan H, Islam S, Tawre M, Pardesi K, Santra MK, Ottoor D (2019) Bioimaging applications of carbon dots (C. dots) and its cystamine functionalization for the sensitive detection of Cr (VI) in aqueous samples. J Fluoresc 29:1381–1392. https://doi.org/10.1007/s10895-019-02448-3
Luo R, Zhang J, Zhuang W, Deng L, Li L, Yu H, Wang J, Huang N, Wang Y (2018) Multifunctional coatings that mimic the endothelium: surface bound active heparin nanoparticles with in situ generation of nitric oxide from nitrosothiols. J Mater Chem B 6:5582–5595. https://doi.org/10.1039/C8TB00596F
Kalsi P (2007) Organic reactions stereochemistry and mechanism (Through Solved Problems). New Age Int. Accessed 12 Sept 2023
Parekh P, Ohno S, Si Y, Lv C, Du B, Ray D, Aswal VK, Bahadur P (2020) Synthesis, aggregation and adsorption behaviour of a thermoresponsive pentablock copolymer. Polym Int 69:1113–1121. https://doi.org/10.1002/pi.5967
Shaikhullina M, Khaliullina A, Gimatdinov R, Butakov A, Chernov V, Filippov A (2020) NMR relaxation and self-diffusion in aqueous micellar gels of pluronic F-127. J Mol Liq 306:112898. https://doi.org/10.1016/j.molliq.2020.112898
Choi JH, Joung YK, Bae JW, Choi JW, Quyen TN, Park KD (2011) Self-assembled nanogel of pluronic-conjugated heparin as a versatile drug nanocarrier. Macromol Res 19:180–188. https://doi.org/10.1007/s13233-011-0214-4
Pomin VH (2016) 1H and 15N NMR analyses on heparin, heparan sulfates and related monosaccharides concerning the chemical exchange regime of the N-sulfo-glucosamine sulfamate proton. Pharmaceuticals 9:58. https://doi.org/10.3390/ph9030058
Nguyen DH, Joung YK, Choi JH, Moon HT, Park KD (2011) Targeting ligand-functionalized and redox-sensitive heparin-Pluronic nanogels for intracellular protein delivery. Biomed Mater 6:055004. https://doi.org/10.1088/1748-6041/6/5/055004
Wang K, Shang T, Zhao J, Zhang L, Zhou L, Deng J, Li X, Wang J (2022) A redox-sensitive coating with disulfide bonds is a promising candidate for surface-modified interventional devices. Mater Today Commun 31:103380. https://doi.org/10.1016/j.mtcomm.2022.103380
Mthembu SN, Sharma A, Albericio F, de la Torre BG (2020) Breaking a couple: disulfide reducing agents. ChemBioChem 21:1947–1954. https://doi.org/10.1002/cbic.202000092
Hegedűs C, Kovács K, Polgár Z, Regdon Z, Szabó É, Robaszkiewicz A, Forman HJ, Martner A, Virág L (2018) Redox control of cancer cell destruction. Redox Biol 16:59–74. https://doi.org/10.1016/j.redox.2018.01.015
Ci L, Huang Z, Liu Y, Liu Z, Wei G, Lu W (2017) Amino-functionalized poloxamer 407 with both mucoadhesive and thermosensitive properties: preparation, characterization and application in a vaginal drug delivery system. Acta Pharm Sinica B 7:593–602. https://doi.org/10.1016/j.apsb.2017.03.002
Bodratti AM, Alexandridis P (2018) Formulation of poloxamers for drug delivery. J Funct Biomater 9:11. https://doi.org/10.3390/jfb9010011
Hou L, Fan Y, Yao J, Zhou J, Li C, Fang Z, Zhang Q (2011) Low molecular weight heparin-all-trans-retinoid acid conjugate as a drug carrier for combination cancer chemotherapy of paclitaxel and all-trans-retinoid acid. Carbohyd Polym 86:1157–1166. https://doi.org/10.1016/j.carbpol.2011.06.008
Yan X, Yang Y, He L, Peng D, Yin D (2017) Gambogic acid grafted low molecular weight heparin micelles for targeted treatment in a hepatocellular carcinoma model with an enhanced anti-angiogenesis effect. Int J Pharm 522:110–118. https://doi.org/10.1016/j.ijpharm.2017.02.051
Joung YK, Jang JY, Choi JH, Han DK, Park KD (2013) Heparin-conjugated pluronic nanogels as multi-drug nanocarriers for combination chemotherapy. Mol Pharm 10:685–693. https://doi.org/10.1021/mp300480v
Thi TTN, Tran TV, Tran NQ, Nguyen CK, Nguyen DH (2017) Hierarchical self-assembly of heparin-PEG end-capped porous silica as a redox sensitive nanocarrier for doxorubicin delivery. Mater Sci Eng, C 70:947–954. https://doi.org/10.1016/j.msec.2016.04.085
Uthaman S, Huh KM, Park I-K (2018) Tumor microenvironment-responsive nanoparticles for cancer theragnostic applications. Biomater Res 22:1–11. https://doi.org/10.1088/1748-6041/6/5/055004
Nguyen DH, Joung YK, Choi JH, Moon HT, Park KD (2011) Targeting ligand-functionalized and redox-sensitive heparin-Pluronic nanogels for intracellular protein delivery. Biomed Mater 6:055004. https://doi.org/10.1088/1748-6041/6/5/055004
Nguyen DH, Choi JH, Joung YK, Park KD (2011) Disulfide-crosslinked heparin-pluronic nanogels as a redox-sensitive nanocarrier for intracellular protein delivery. J Bioact Compat Polym 26:287–300. https://doi.org/10.1177/088391151140603
Chen W, Zheng M, Meng F, Cheng R, Deng C, Feijen J, Zhong Z (2013) In situ forming reduction-sensitive degradable nanogels for facile loading and triggered intracellular release of proteins. Biomacromol 14:1214–1222. https://doi.org/10.1021/bm400206m
Elkassih SA, Kos P, Xiong H, Siegwart DJ (2019) Degradable redox-responsive disulfide-based nanogel drug carriers via dithiol oxidation polymerization. Biomater Sci 7:607–617. https://doi.org/10.1039/C8BM01120F
Gurbuz HA, Durukan AB, Sevim H, Ergin E, Gurpinar A, Yorgancioglu C (2013) Heparin toxicity in cell culture: a critical link in translation of basic science to clinical practice. Blood Coag Fibrinol 24:742–745. https://doi.org/10.1097/MBC.0b013e3283629bbc
Eidi H, Joubert O, Attik G, Duval RE, Bottin M, Hamouia A, Maincent P, Rihn B (2010) Cytotoxicity assessment of heparin nanoparticles in NR8383 macrophages. Int J Pharm 396:156–165. https://doi.org/10.1016/j.ijpharm.2010.06.006
Funding
This study was funded by the 562 Program—Basic Science Development in the fields of life, earth, and marine science in the period of 2017–2025 orienting to 2030, under grant number ĐTĐL.CN-53/21.
Author information
Authors and Affiliations
Contributions
Nguyen T. Huong, Nguyen T.N. Hoi, Vu M. Thanh: Methodology, Investigation, Funding acquisition. Mac D. Hung, Nguyen V. Hung, Le D. Anh: Formal analysis. Vu T. Dong, Ly Q. Vuong, Le M. Tri: Formal analysis, Supervision.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Conflict of interest/Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huong, N.T., Hoi, N.T.N., Hung, M.D. et al. A redox-responsive delivery system for paclitaxel based on heparin—pluronic F127 nanogel. J Nanopart Res 25, 191 (2023). https://doi.org/10.1007/s11051-023-05841-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-023-05841-z