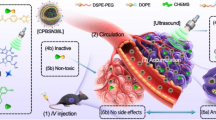
Abstract
Ultrasound-mediated delivery has garnered attention as a noninvasive modality for localized drug delivery in cancer. Doxorubicin, a chemotherapeutic drug, shows low penetration, nonspecific distribution, and uncontrolled release. We have synthesized a hydrophobic doxorubicin (hDox)-loaded palmitoyl-modified glycol chitosan amphiphile (PmGCA) polymer-based nanotheranostic drug delivery system (hDox-NDS) for ultrasound (US)-mediated release. PmGCA encapsulates 210 μg/mL hDox in 90 ± 15 nm cationic micelles. The reappearance of the Dox fluorescence peak after 2 MHz US exposure confirms the encapsulated drug release.
hDox-NDS display a considerable drug release (~30 to 50%) over 72 h, following the Weibull model. Fluorescence microscopy shows a higher uptake of hDox-NDS (~10%) in rhabdomyosarcoma (RD) cells compared to DoxHCl. Ultrasound exposure significantly (p < 0.001) enhances the anticancer effect of hDox-NDS on RD cells, as shown by its lower IC50 value of 1.7 ± 0.05 μM compared to that of DoxHCl (2.62 ± 1.2 μM). In vivo optical imaging shows significantly lower fluorescence in the heart (~84%) and liver (~59%) compared to DoxHCl, thereby reducing side effects. This nanotheranostic hDox-NDS has been considered an effective drug delivery system, offering enhanced therapeutic effects in cancer treatment.









Similar content being viewed by others
References
Ladju RB, Ulhaq ZS, Soraya GV (2022) Nanotheranostics: a powerful next-generation solution to tackle hepatocellular carcinoma. World J Gastroenterol 28:176
Afadzi M, Myhre OF, Yemane PT et al (2020) Effect of acoustic radiation force on the distribution of nanoparticles in solid tumors. IEEE Trans Ultrason Ferroelectr Freq Control 68:432–445
Kim D, Lee JH, Moon H et al (2021) Development and evaluation of an ultrasound-triggered microbubble combined transarterial chemoembolization (TACE) formulation on rabbit VX2 liver cancer model. Theranostics 11:79
Young RC, Ozols RF, Myers CE (1981) The anthracycline antineoplastic drugs. N Engl J Med 305:139–153
Melim C, Magalhães M, Santos AC et al (2022) Nanoparticles as phytochemical carriers for cancer treatment: news of the last decade. Expert Opin Drug Deliv 19:179–197
Gabizon A, Shmeeda H, Barenholz Y (2003) Pharmacokinetics of pegylated liposomal doxorubicin. Clin Pharmacokinet 42:419–436
Hekmatirad S, Moloudizargari M, Moghadamnia AA et al (2021) Inhibition of exosome release sensitizes U937 cells to PEGylated liposomal doxorubicin. Front Immunol 12:2008
Hilger RA, Richly H, Grubert M et al (2005) Pharmacokinetics (PK) of a liposomal encapsulated fraction containing doxorubicin and of doxorubicin released from the liposomal capsule after intravenous infusion of Caelyx/Doxil. Int J Clin Pharmacol Ther 43:588–589
Liu L, Kshirsagar PG, Gautam SK et al (2022) Nanocarriers for pancreatic cancer imaging, treatments, and immunotherapies. Theranostics 12:1030
Entzian K, Aigner A (2021) Drug delivery by ultrasound-responsive nanocarriers for cancer treatment. Pharmaceutics 13:1135
Mohan P, Rapoport N (2010) Doxorubicin as a molecular nanotheranostic agent: effect of doxorubicin encapsulation in micelles or nanoemulsions on the ultrasound-mediated intracellular delivery and nuclear trafficking. Mol Pharm 7:1959–1973
Mi P (2020) Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics 10:4557
Behera SK, Mohanty ME, Mohapatra M (2021) A fluorescence study of the interaction of anticancer drug molecule doxorubicin hydrochloride in pluronic P123 and F127 micelles. J Fluoresc 31:17–27
Afshari AR, Sanati M, Mollazadeh H et al (2022) Nanoparticle-based drug delivery systems in cancer: a focus on inflammatory pathways. Semin Cancer Biol. https://doi.org/10.1016/j.semcancer.2022.01.008
Xia H, Yang D, He W et al (2021) Ultrasound-mediated microbubbles cavitation enhanced chemotherapy of advanced prostate cancer by increasing the permeability of blood-prostate barrier. Transl Oncol 14:101177
Zia N, Iqbal Z, Raza A et al (2022) Glycol-chitosan-based Technetium-99m-loaded multifunctional nanomicelles: synthesis, evaluation, and in vivo biodistribution. Nanomaterials 12:2198. https://doi.org/10.3390/nano12132198
Uchegbu IF, Sadiq L, Arastoo M et al (2001) Quaternary ammonium palmitoyl glycol chitosan—a new polysoap for drug delivery. Int J Pharm 224:185–199
Lalatsa A, Schätzlein AG, Garrett NL et al (2015) Chitosan amphiphile coating of peptide nanofibres reduces liver uptake and delivers the peptide to the brain on intravenous administration. J Controlled Release 197:87–96. https://doi.org/10.1016/j.jconrel.2014.10.028
Min HS, You DG, Son S et al (2015) Echogenic glycol chitosan nanoparticles for ultrasound-triggered cancer Theranostics. Theranostics 5:1402–1418. https://doi.org/10.7150/thno.13099
Zhou X, Guo L, Shi D et al (2019) Biocompatible chitosan nanobubbles for ultrasound-mediated targeted delivery of doxorubicin. Nanoscale Res Lett 14:24. https://doi.org/10.1186/s11671-019-2853-x
Meng D, Guo L, Shi D et al (2019) Charge-conversion and ultrasound-responsive O-carboxymethyl chitosan nanodroplets for controlled drug delivery. Nanomed 14:2549–2565
Kanwal U, Bukhari NI, Rana NF et al (2019) Doxorubicin-loaded quaternary ammonium palmitoyl glycol chitosan polymeric nanoformulation: uptake by cells and organs. Int J Nanomedicine 14:1–15
Wei T, Chen C, Liu J et al (2015) Anticancer drug nanomicelles formed by self-assembling amphiphilic dendrimer to combat cancer drug resistance. Proc Natl Acad Sci 112:2978–2983
Khan MA, Ansari MM, Arif ST et al (2021) Eplerenone nanocrystals engineered by controlled crystallization for enhanced oral bioavailability. Drug Deliv 28:2510–2524
Ovais M, Nadhman A, Khalil AT et al (2017) Biosynthesized colloidal silver and gold nanoparticles as emerging leishmanicidal agents: an insight. Nanomed 12:2807–2819
Li K, Li P, Jia Z et al (2018) Enhanced fluorescent intensity of magnetic-fluorescent bifunctional PLGA microspheres based on Janus electrospraying for bioapplication. Sci Rep 8:1–11
Rehman M, Raza A, Khan JA, Zia MA (2021) Laser responsive cisplatin-gold nano-assembly synergizes the effect of cisplatin with compliance. J Pharm Sci 110:1749–1760
Mannaris C, Teo BM, Seth A et al (2018) Gas-stabilizing gold nanocones for acoustically mediated drug delivery. Adv Healthc Mater 7:1800184
Sarwar U, Naeem M, Nurjis F, et al (2022) Ultrasound-mediated in vivo biodistribution of coumarin-labeled sorafenib-loaded liposome-based nanotheranostic system. Nanomed 17:1909–1927
Salgarella AR, Zahoranová A, Šrámková P et al (2018) Investigation of drug release modulation from poly (2-oxazoline) micelles through ultrasound. Sci Rep 8:1–13
Nelson TR, Fowlkes JB, Abramowicz JS, Church CC (2009) Ultrasound biosafety considerations for the practicing sonographer and sonologist. J Ultrasound Med 28:139–150. https://doi.org/10.7863/jum.2009.28.2.139
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Lefort CT, Kim M (2010) Human T lymphocyte isolation, culture and analysis of migration in vitro. JoVE J Vis Exp e2017
Jawaid P, Rehman MU, Hassan MA et al (2016) Effect of platinum nanoparticles on cell death induced by ultrasound in human lymphoma U937 cells. Ultrason Sonochem 31:206–215. https://doi.org/10.1016/j.ultsonch.2015.12.013
Esmaeilzadeh-Gharedaghi E, Faramarzi MA, Amini MA et al (2012) Effects of processing parameters on particle size of ultrasound prepared chitosan nanoparticles: an artificial neural networks study. Pharm Dev Technol 17:638–647
Uchegbu IF, Breznikar J, Zaffalon A et al (2021) Polymeric micelles for the enhanced deposition of hydrophobic drugs into ocular tissues, without plasma exposure. Pharmaceutics 13:744
Gnapareddy B, Reddy Dugasani S, Ha T et al (2015) Chemical and physical characteristics of doxorubicin hydrochloride drug-doped salmon DNA thin films. Sci Rep 5:1–9
Huang K-Y, He H-X, He S-B et al (2019) Gold nanocluster-based fluorescence turn-off probe for sensing of doxorubicin by photoinduced electron transfer. Sens Actuators B Chem 296:126656
Chooi KW, Carlos MIS, Soundararajan R et al (2014) Physical characterisation and long-term stability studies on quaternary ammonium palmitoyl glycol chitosan (GCPQ)—a new drug delivery polymer. J Pharm Sci 103:2296–2306
Lu Y, Zhang E, Yang J, Cao Z (2018) Strategies to improve micelle stability for drug delivery. Nano Res 11:4985–4998. https://doi.org/10.1007/s12274-018-2152-3
Lu D, Wen X, Liang J et al (2009) A pH-sensitive nano drug delivery system derived from pullulan/doxorubicin conjugate. J Biomed Mater Res B Appl Biomater 89B:177–183. https://doi.org/10.1002/jbm.b.31203
Choi J-S, Park J-W, Seu Y-B, Doh K-O (2021) Enhanced efficacy of folate-incorporated cholesteryl doxorubicin liposome in folate receptor abundant cancer cell. J Drug Deliv Sci Technol 62:102385
Wu P, Jia Y, Qu F et al (2017) Ultrasound-responsive polymeric micelles for sonoporation-assisted site-specific therapeutic action. ACS Appl Mater Interfaces 9:25706–25716
Lalatsa A, Schatzlein AG, Uchegbu IF (2014) Strategies to deliver peptide drugs to the brain. Mol Pharm 11:1081–1093
Izci M, Maksoudian C, Manshian BB, Soenen SJ (2021) The use of alternative strategies for enhanced nanoparticle delivery to solid tumors. Chem Rev 121:1746–1803
Herdiana Y, Wathoni N, Shamsuddin S, Muchtaridi M (2022) Drug release study of the chitosan-based nanoparticles. Heliyon 8:e08674. https://doi.org/10.1016/j.heliyon.2021.e08674
Foroozandeh P, Aziz AA (2018) Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res Lett 13:1–12
Snipstad S, Berg S, Mørch Ý et al (2017) Ultrasound improves the delivery and therapeutic effect of nanoparticle-stabilized microbubbles in breast cancer xenografts. Ultrasound Med Biol 43:2651–2669
Xiong R, Xu RX, Huang C et al (2021) Stimuli-responsive nanobubbles for biomedical applications. Chem Soc Rev 50:5746–5776
Champion JA, Walker A, Mitragotri S (2008) Role of particle size in phagocytosis of polymeric microspheres. Pharm Res 25:1815–1821
Fang C, Kievit FM, Cho Y-C et al (2012) Effect of cationic side-chains on intracellular delivery and cytotoxicity of pH sensitive polymer–doxorubicin nanocarriers. Nanoscale 4:7012–7020
Jiang LQ, Wang TY, Webster TJ et al (2017) Intracellular disposition of chitosan nanoparticles in macrophages: intracellular uptake, exocytosis, and intercellular transport. Int J Nanomedicine 12:6383
Alqahtani MS, Syed R, Alshehri M (2020) Size-dependent phagocytic uptake and immunogenicity of gliadin nanoparticles. Polymers 12:2576
Vonarbourg A, Passirani C, Saulnier P, Benoit J-P (2006) Parameters influencing the stealthiness of colloidal drug delivery systems. Biomaterials 27:4356–4373
Nittayacharn P, Yuan H-X, Hernandez C et al (2019) Enhancing tumor drug distribution with ultrasound-triggered nanobubbles. J Pharm Sci 108:3091–3098
Fant C, Lafond M, Rogez B et al (2019) In vitro potentiation of doxorubicin by unseeded controlled non-inertial ultrasound cavitation. Sci Rep 9:15581. https://doi.org/10.1038/s41598-019-51785-7
Liang H-D, Lu QL, Xue S-A et al (2004) Optimisation of ultrasound-mediated gene transfer (sonoporation) in skeletal muscle cells. Ultrasound Med Biol 30:1523–1529. https://doi.org/10.1016/j.ultrasmedbio.2004.08.021
Zhang Z, Wang Y, Ma Q et al (2021) Biomimetic carrier-free nanoparticle delivers digoxin and doxorubicin to exhibit synergetic antitumor activity in vitro and in vivo. Chem Eng J 406:126801
Liu D, Mori A, Huang L (1992) Role of liposome size and RES blockade in controlling biodistribution and tumor uptake of GM1-containing liposomes. Biochim Biophys Acta BBA - Biomembr 1104:95–101. https://doi.org/10.1016/0005-2736(92)90136-A
Acknowledgements
Chemicals and equipment used in the research work are supported through the PAK-NORWAY Institutional Cooperation Program, PK3004, COMSTECH-TWAS (12-198 RG/PHA/AS_C-UNESCO FR: 3240270874), and Pakistan Science Foundation (PSF/Res/C-NILOP/Med (330). The authors are grateful to the Pakistan Institute of Applied Sciences (PIEAS) and the National Institute of Health (NIH) for technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 153 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saeed, S., Sarwar, U., Yasinzai, M. et al. Glycol chitosan amphiphile nanotheranostic system for ultrasound-mediated localized release and biodistribution of doxorubicin. J Nanopart Res 25, 194 (2023). https://doi.org/10.1007/s11051-023-05835-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-023-05835-x