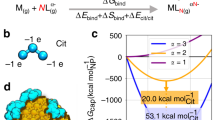
Abstract
It is known that citrate capped gold nanoparticles (Ct-AuNP) aggregate when an electrolyte is added to the medium due to the electrostatic screening produced by the cations on the nanoparticle surface. Small cations are mostly employed when studying this effect. Instead, here we use the large monovalent cations coming from tetrabutylammonium chloride (Bu4N+Cl-), tetrapropylammonium chloride (Pr4N+Cl-) and tetraethylammonium chloride (Et4N+Cl-) in order to induce the aggregation of Ct-AuNP of 20 nm of hydrodynamic diameter. Then, we have followed the aggregation kinetics by means of dynamic light scattering and ultraviolet-visible spectroscopy. Also, we have measured the zeta potential and taken transmission electron microscopy images. We show that these large cations produce a quick aggregation which is stronger as larger is the cation. Thus, the results indicate that larger cations screen more efficiently the Ct-AuNP surface which can be ascribed to the weaker hydration of larger cations. On the other hand, the greater destabilising effect produced by larger cations is in agreement with the direct Hofmeister series.
Graphical abstract






Similar content being viewed by others
References
Kohout C, Santi C, Polito L (2018) Anisotropic gold nanoparticles in biomedical applications. Int J Mol Sci 19:3385. https://doi.org/10.3390/ijms19113385
Siddiq AM, Thangam R, Madhan B, Sayem Alam M (2019) Counterion coupled (COCO) gemini surfactant capped Ag/Au alloy and Ag@Aun core-shell nanoparticles for cancer therapy. RSC Adv 9:37830–37845. https://doi.org/10.1039/c9ra06494j
Siddiq AM, Thangam R, Madhan B, Sayem Alam M (2019) Green (gemini) surfactant mediated gold nanoparticles green synthesis: effect on triple negative breast cancer cells. Nano Struct Nano-Objects 19:100373. https://doi.org/10.1016/j.nanoso.2019.100373
Mbambo AT, Kruger HG, Mdluli PS, Madikizela LM (2019) Fabrication and application of a gold nanoparticle based colorimetric device for the determination of NaCl in seawater and estuarine water. J Nanopart Res 21:135. https://doi.org/10.1007/s11051-019-4579-1
Grueso, E.; Giráldez-Pérez, R.M.; Prado-Gotor, R. Recent advances in the design of colorimetric sensors based on gold nanoparticles. In Advanced Nanomaterials; Ikhmayies, S.J., Ed.; Springer: Cham, Swiss, 2022, pp 445-495. https://doi.org/10.1007/978-3-031-11996-5_16
Bai X, Wang Y, Song Z, Feng Y, Chen Y, Zhang D, Feng L (2020) The basic properties of gold nanoparticles and their applications in tumor diagnosis and treatment. Int J Mol Sci 21:2480. https://doi.org/10.3390/ijms21072480
Thakuria A, Kataria B, Gupta D (2021) Nanoparticle-based methodologies for targeted drug delivery – an insight. J Nanopart Res 23:87. https://doi.org/10.1007/s11051-021-05190-9
Grys D-B, de Nijs B, Salmon AR, Huang J, Wang W, Chen W-H, Scherman OA, Baumberg JJ (2020) Citrate coordination and bridging of gold nanoparticles: The role of gold adatoms in AuNP aging. ACS Nano 14:8689–8696. https://doi.org/10.1021/acsnano.0c03050
Collado-González M, Fernández Espín V, Montalbán MG, Pamies R, Hernández Cifre JG, Díaz Baños FG, Víllora G, García de la Torre J (2015) Aggregation behaviour of gold nanoparticles in presence of chitosan. J Nanopart Res 17:268. https://doi.org/10.1007/s11051-015-3069-3
Herrera Robalino D, Durán del Amor MM, Almagro Gómez CM, Hernández Cifre JG (2021) Aggregation of gold nanoparticles in presence of the thermoresponsive cationic diblock copolymer PNIPAAM48-b-PAMPTMA6. Polymers 13:4066. https://doi.org/10.3390/polym13234066
Pamies R, Hernández Cifre JG, Fernández Espín V, Collado-González MM, Díaz Baños FG, García de la Torre J (2014) Aggregation behaviour of gold nanoparticles in saline aqueous media. J Nanopart Res 16:2376. https://doi.org/10.1007/s11051-014-2376-4
Christau S, Moeller T, Koehler R, von Klitzing R (2017) Salt-induced aggregation of negatively charged gold nanoparticles confined in a polymer brush matrix. Macromolecules 50:7333–7343. https://doi.org/10.1021/acs.macromo.7b00866
Barreto A, Luis LG, Girão AV, Trindade T, Soares AMVM, Oliveira M (2015) Behavior of colloidal gold nanoparticles in different ionic strength media. J Nanopart Res 17:493. https://doi.org/10.1007/s11051-015-3302-0
Alba-Molina D, Martín-Romero MT, Camacho L, Giner-Casares JJ (2017) Ion-mediated aggregation of gold nanoparticles for light-induced heating. Appl Sci 7:916. https://doi.org/10.3390/app7090916
Pfeiffer C, Rehbock C, Hühn D, Carrillo-Carrión C, de Aberasturi DJ, Merk V, Barcikowski S, Parak WJ (2014) Interaction of colloidal nanoparticles with their local environment: the (ionic) nanoenvironment around nanoparticles is different from bulk and determines the physico-chemical properties of the nanoparticles. J R Soc Interface 11:20130931. https://doi.org/10.1098/rsif.2013.0931
Liu B, Kelly EY, Liu J (2014) Cation-size-dependent DNA adsorption kinetics and packing density on gold nanoparticles: an opposite trend. Langmuir 30:13228–13234. https://doi.org/10.1021/la503188h
Oncsik T, Trefalt G, Borkovec M, Szilagyi I (2015) Specific ion effects on particle aggregation induced by monovalent salts within the Hofmeister series. Langmuir 31:3799–3807. https://doi.org/10.1021/acs.langmuir.5b00225
Perera GS, Yang G, Nettles CB II, Pérez F, Hollis TK, Zhang D (2016) Counterion effects on electrolyte interactions with gold nanoparticles. J Phys Chem C 120:23604–23612. https://doi.org/10.1021/acs.jpcc.6b07885
Hu S, Huang P-JJ, Wang J, Liu J (2020) Dissecting the effect of salt for more sensitive label-free colorimetric detection of DNA using gold nanoparticles. Anal Chem 92:13354–13360. https://doi.org/10.1021/acs.analchem.0c02688
Jagtap NR, Shelke VA, Nimase MS, Jadhav SM, Shankarwar SG, Chondhekar TK (2012) Electrochemical synthesis of tetra alkyl ammonium salt stabilized gold nanoparticles. Synthesis and reactivity in inorganic, metal-organic and nano-metal chemistry 42:1369–1374. https://doi.org/10.1080/15533174.2012.680124
Morita T, Yada S, Yoshimura T (2023) Catalytic activity of gold nanoparticles protected by quaternary ammonium salt-based gemini surfactants with different spacer structures. Phys Chem Chem Phys 25:16288–16293. https://doi.org/10.1039/d3cp01116j
Gregory KP, Elliot GR, Robertson H, Kumar A, Wanless EJ, Webber GB, Craig VSJ, Andersson GG, Page AJ (2022) Understanding specific ion effects and the Hofmeister series. Phys Chem Chem Phys 24:12682–12718. https://doi.org/10.1039/d2cp00847e
Suarasan S, Focsan M, Maniu D, Astilean S (2013) Gelatin-nanogold bioconjugates as effective plasmonic platforms for SERS detection and tagging. Colloid Surf B-Biointerfaces 103:475–481. https://doi.org/10.1016/j.colsurfb.2012.10.046
Zheng T, Bott S, Huo Q (2016) Techniques for accurate sizing of gold nanoparticles using dynamic light scattering with particular application to chemical and biological sensing based on aggregate formation. ACS Appl Mater Interfaces 8:21585–21594. https://doi.org/10.1021/acsami.6b06903
Marcus Y (2008) Tetraalkylammonium ions in aqueous and non-aqueous solutions. J Solut Chem 37:1071–1098. https://doi.org/10.1007/s10953-008-9291-1
Verwey EJW, Overbeek JTG (1948) Theory of the stability of lyophobic colloids. Elsevier, New York
Olivier BJ, Sorensen CM (1990) Variable aggregation rates in colloidal gold: kernel homogeneity dependence on aggregant concentration. Phys Rev A 41:2093–2100 https://journals.aps.org/pra/pdf/10.1103/PhysRevA.41.2093
Wilcoxon JP, Martin JE, Schaefer DW (1989) Aggregation in colloidal gold. Phys Rev A 39:2675–2688 https://journals.aps.org/pra/pdf/10.1103/PhysRevA.39.2675
Liu X, Wazne M, Han Y, Christodoulatos C, Jasinkiewicz KL (2010) Effects of natural organic matter on aggregation kinetics of boron nanoparticles in monovalent and divalent electrolytes. J Colloid Interface Sci 348:101–107. https://doi.org/10.1016/j.jcis.2010.04.036
Slusher JT, Cummings PT (1997) Molecular simulation study of tetraalkylammonium halides. 1. Solvation structure and hydrogen bonding in aqueous solutions. J Phys Chem B 101:3818–3826. https://doi.org/10.1021/jp963304+
Funding
This study was supported by Fundación Séneca (Comunidad Autónoma de la Región de Murcia, Spain) with grant number 20933/PI/18 and European Union “NextGenerationEU”/PRTR" through the project TED2021-130334B-I00.
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology, J.A.O. and J.G.H.-C.; investigation, C.M.A.-G., J.A.O. and J.G.H.-C.; visualization, C.M.A.-G. and J.G.H.-C.; formal analysis, C.M.A.-G., J.A.O. and J.G.H.-C.; validation and supervision, J.A.O., J.G.T. and J.G.H.-C.; writing—original draft preparation, J.G.H.-C.; writing—review and editing J.G.T. and J.G.H.-C.; resources and project administration J.G.T. and J.G.H.-C.; funding acquisition, J.G.T. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(XLSX 78 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Almagro-Gómez, C.M., Ortuño, J.Á., de la Torre, J.G. et al. Effect of large ammonium cations on the aggregation kinetics of citrate capped gold nanoparticles. J Nanopart Res 25, 175 (2023). https://doi.org/10.1007/s11051-023-05825-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-023-05825-z