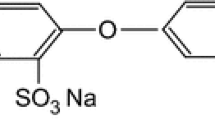
Abstract
The adsorption mechanism of superplasticizer molecules on the surface of ettringite was analyzed by molecular dynamic (MD) simulation, and the reasons for the inhibition of ettringite crystal growth by comb-shaped polycarboxylate ether-based (PCE) molecules were revealed. Three methacrylate (MPEG)-based PCEs with different anionic charge amounts were examined in vacuum and solution, respectively. The simulation results of different PCE models were compared, and it was found that there were differences in adsorption conformation, adsorption stability, water density at the interface, migration capacity of water molecules near the surface, and stability of water molecules in PCE membranes. The study found that the interaction between PCEs and ettringite depends on the amount of anion charge. Adding an appropriate amount of anion charge can enhance the interfacial binding energy and make the adsorption of PCEs on the surface of ettringite more compact. However, excessive negative anionic charge at high salt concentrations in solution simulations can change the polymer conformation of the group. By calculating the interfacial density distribution parameters of water molecules, the distance function of PCE molecules and the adsorption on the surface of the model, the MSD and diffusion coefficient of water molecules between layers, and the self-diffusion coefficient and surface adsorption strength of PCEs, it is found that the presence of PCEs not only disturbs the dense water layer above the surface of ettringite, reduces the water density, but also weakens the fluidity of water molecules on the surface of ettringite, slows down the ion exchange rate in the system, and thus inhibits the growth of ettringite crystals.











Similar content being viewed by others
Data availability
Some or all data, models, or code that support the findings of this study are available from the corresponding author upon reasonable request.
References
Cheung J, Jeknavorian A, Roberts L, Silva D (2011) Impact of admixtures on the hydration kinetics of Portland cement. Cem Concr Res 41(12):1289–1309
Plank J, Hirsch C (2007) Impact of zeta potential of early cement hydration phases on superplasticizer adsorption. Cem Concr Res 37:537–542
Jansen D, Neubauer J, Goetz-Neunhoeffer F, Haerzschel R, Hergeth WD (2012) Change in reaction kinetics of a Portland cement caused by a superplasticizer-calculation of heat flow curves from XRD data. Cem Concr Res 42(2):327–332
Kreppelt F, Weibel M, Zampini D, Romer M (2002) Influence of solution chemistry on the hydration of polished clinker surfaces – a study of different types of polycarboxylic acidbased admixtures. Cem Concr Res 32(2):187–198
Schönlein M, Plank J (2018) Influence of PCEs kind and dosage on ettringite crystallization performed under terrestrial and microgravity conditions. J Am Ceram Soc 101:3575–3584
Dalas F, Pourchet S, Rinaldi D et al (2015) Modification of the rate of formation and surface area of ettringite by polycarboxylate ether superplasticizers during early C3A-CaSO4 hydration. Cem Concr Res 69:105–113
Meier MR, Rinkenburger A, Plank J (2016) Impact of different types of polycarboxylate superplasticisers on spontaneous crystallization of ettringite. Adv Cem Res 28(5):310–319
Meier MR, Plank J (2016) Crystal growth of [Ca3Al(OH)6·12H2O]2·(SO4)3·2H2O (ettringite) under microgravity: on the impact of anionicity of polycarboxylate comb polymers. J Cryst Growth 446:92–102
Zingg A, Holzer L, Kaech A, Winnefeld F, Pakusch J, Becker S, Gauckler L (2008) The microstructure of dispersed and non-dispersed fresh cement pastes – new insight by cryo-microscopy. Cem Concr Res 38(4):522–529
Carrillo JMY, Dobrynin AV (2007) Molecular dynamics simulations of polyelectrolyte adsorption. Langmuir 23:2472–2482
Turesson M, Labbez C, Nonat A (2011) Calcium mediatedpolyelectrolyte adsorption on like-charged surfaces. Langmuir 27:13572–13581
Turesson M, Jönsson B, Labbez C (2012) Coarse-graining intermolecular interactions in dispersions of highly charged colloids. Langmuir 28:4926–4930
Turesson M, Nonat A, Labbez C (2014) Stability of negatively charged platelets in calcium-rich anionic copolymer solutions. Langmuir 30:6713–6720
Sun H, Ren P, Fried JR (1998) The COMPASS force field: parameterization and validation for phosphazenes. Comput Theor Polym Sci 8:229–246
Hartman MR, Berliner R (2006) Investigation of the structure of ettringite by time-of-flight neutron powder diffraction techniques. Cem Concr Res 36(2):364–370
Hou DS, Li T, Han QH, Zhang JR (2018) Insight on the sodium and chloride ions adsorption mechanism on the ettringite crystal: structure, dynamics and interfacial interaction. Comput Mater Sci 153:479–492
Zhao HX, Wang YW, Yang Y, Shu X, Yan H, Ran QP (2017) Effect of hydrophobic groups on the adsorption conformation of modified polycarboxylate superplasticizer investigated by molecular dynamics simulation. Appl Surf Sci 407:8–15
Fan QC, Meng X, Li ZD, Ma GY, Wang ZP, Zhang K, He C, Meng D (2022) Experiment and molecular dynamics simulation of functionalized cellulose nanocrystals as reinforcement in cement composites. Constr Build Mater 341:127879
Lange A, Hirata T, Plank J (2014) Influence of the HLB value of polycarboxylate superplasticizers on the flow behavior of mortar and concrete. Cem Concr Res 60:45–50
Hou DS, Li T (2018) Influence of aluminates on the structure and dynamics of water and ions in the nanometer channel of calcium silicate hydrate (C–S–H) gel. Phys Chem Chem Phys 20:2373–2387
Heinz H, Vaia RA, Farmer B, Naik RR (2008) Accurate simulation of surfaces andinterfaces of face-centered cubic metals using 12–6 and 9–6 Lennard-Jones potentials. J Phys Chem C 112:17281–17290
Wang ZQ, Gong YL, Jing C, Huang HJ, Li HR, Zhang ST, Gao F (2016) Synthesis of dibenzotriazole derivatives bearing alkylene linkers as corrosion inhibitors for copper in sodium chloride solution: a new thought for the design of organic inhibitors. Corros Sci 113:64–77
Andersen HC (1980) Molecular dynamics simulations at constant pressure and/or temperature. J Chem Phys 72:2384–2393
Yoshioka K, Tazawa E, Kawai K, Enohata T (2002) Adsorption characteristics of superplasticizers on cement component minerals. Cem Concr Res 32(10):1507–1513
Zingg A, Winnefeld F, Holzer L, Pakusch J, Becker S, Gauckler L (2008) Adsorption of polyelectrolytes and its influence on the rheology zeta potential, and microstructure of various cement and hydrate phases. J Colloid Interface Sci 23:301–312
Shu X, Ran QP, Liu JP, Zhao HX, Zhang Q, Wang XM, Yang Y, Liu JZ (2016) Tailoring the solution conformation of polycarboxylate superplasticizer toward the improvement of dispersing performance in cement paste. Constr Build Mater 116:289–298
Ran QP, Somasundaran P, Miao CW, Liu JP, Wu SS, Shen J (2010) Adsorption mechanism of comb polymer dispersants at the cement/water interface. J Disper Sci Technol 31:790–798
Sanchez F, Zhang L (2008) Molecular dynamics modeling of the interface between surface functionalized graphitic structures and calcium–silicate–hydrate: interaction energies, structure, and dynamics. J Colloid Interface Sci 323(2):349–358
Hou DS, Zhang QG, Wang MH, Zhang JH, Wang P, Ge YM (2019) Molecular dynamics study on water and ions on the surface of graphene oxide sheet: Effects of functional groups. Comput Mater Sci 167:237–247
Wang P, Qiao G, Guo YP, Zhang Y, Hou DS, Jin ZQ, Zhang JR, Wang MH, Hu XX (2020) Molecular dynamics simulation of the interfacial bonding properties between graphene oxide and calcium silicate hydrate. Constr Build Mater 260:119927
Wang YW, Teraoka I, Hansen FY, Peters GH, Hassager O (2011) Mean span dimensions of ideal polymer chains containing branches and rings. Macromol 44:403–412
Salles F, Jobic H, Devic T, Llewellyn PL, Serre C, Férey G, Maurin G (2010) Self and transport diffusivity of CO2 in the metal-organic framework MIL-47(V) explored by quasi-elastic neutron scattering experiments and molecular dynamics simulations. ACS Nano 4:143–152
Kirby GH, Lewis JA (2004) Comb polymer architecture effects on the rheological property evolution of concentrated cement suspensions. J Am Ceram Soc 87:1643–1652
Yamada K, Takahashi T, Hanehara S, Matsuhisa M (2000) Effects of the chemical structure on the properties of polycarboxylate-type superplasticizer. Cem Concr Res 30:197–207
Wang X, Liu L, Wang P, Li W, Zhang J, Yan YG (2014) How the inhibition performance is affected by inhibitor concentration: a perspective from microscopic adsorption behavior. Ind Eng Chem Res 53:16785–16792
Ran QP, Somasundaran P, Miao CW, Liu JP, Wu SS, Shen J (2009) Effect of the length of the side chains of comb-like copolymer dispersants on dispersion and rheological properties of concentrated cement suspensions. J Colloid Interface Sci 326:624–633
Zhang Q, Ran QP, Zhao HX, Shu X, Yang Y (2017) Effect of counter-ions on comb-like polycarboxylate conformation in aqueous solutions. J Disper Sci Technol 38:721–728
Acknowledgements
The authors acknowledge the Supercomputing Center of Dalian University of Technology for providing computing resources.
Funding
This study received financial support from the Teaching research project of Qingdao Agricultural University, High-level Talent Scientific Research Fund project of Qingdao Agricultural University (1114324) and postgraduate innovation program of Qingdao Agricultural University (Grant Nos. QNYCX21022 and QNYCX22050).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meng, D., Meng, X., Fan, Q. et al. Effect of anionic charge quantity on adsorption properties of PCE molecules on ettringite surface: a molecular dynamic simulation method. J Nanopart Res 25, 161 (2023). https://doi.org/10.1007/s11051-023-05798-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-023-05798-z