Abstract
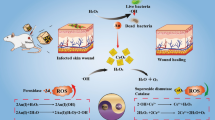
Overuse of antibiotics has led to the emergency of vancomycin-resistant Enterococcus (VRE), considered one of the leading causes of severe diseases and even death. The phototherapy strategy of combined photothermal therapy (PTT) and photodynamic therapy (PDT) has achieved considerable attention since it could effectively enhance synergistic efficacy and avoid new drug resistance. In this paper, a kind of hybrid nanocomposite was fabricated and applied in the phototherapy-based combination strategy against VRE pathogens, in which graphene oxide (GO) not only covalent conjugated with vancomycin (Van) but also loaded photosensitizer phthalocyanine (Pc) via π–π stacking interaction. Outstanding PTT and PDT effects were observed in the as-prepared nanocomposite (GO@Van@Pc), giving almost 2–3 logs of bacterial reduction in vitro. Similar data were also obtained when GO@Van@Pc was used to treat VRE-infected BALB/C mice, exhibiting obvious infection regression and accelerated wound healing with negligible damage to normal cells and major organs. Therefore, the as-prepared GO@Van@Pc integrating PTT and PDT has great potential in the phototherapy of drug-resistant pathogens.
Graphical Abstract









Similar content being viewed by others
References
Eisenberger D, Tuschak C, Werner M, Bogdan C, Bollinger T, Hossain H, Friedrich P, Hussein Z, Pohlmann C, Wurstl B, Nickel S, Lehner-Reindl V, Holler C, Liebl B, Valenza G (2020) Whole-genome analysis of vancomycin-resistant Enterococcus faecium causing nosocomial outbreaks suggests the occurrence of few endemic clonal lineages in Bavaria, Germany. J Antimicrob Chemother 75(6):1398–1404. https://doi.org/10.1093/jac/dkaa041
Kaur J, Cao X, Abutaleb NS, Elkashif A, Graboski AL, Krabill AD, AbdelKhalek AH, An W, Bhardwaj A, Seleem MN, Flaherty DP (2020) Optimization of acetazolamide-based scaffold as potent inhibitors of vancomycin-resistant Enterococcus. J Med Chem 63(17):9540–9562. https://doi.org/10.1021/acs.jmedchem.0c00734
Zou Z, Sun J, Li Q, Pu Y, Liu J, Sun R, Wang L, Jiang T (2020) Vancomycin modified copper sulfide nanoparticles for photokilling of vancomycin-resistant Enterococci bacteria. Colloids Surf B Biointerfaces 189:110875. https://doi.org/10.1016/j.colsurfb.2020.110875
Jelinkova P, Splichal Z, Jimenez AMJ, Haddad Y, Mazumdar A, Sur VP, Milosavljevic V, Kopel P, Buchtelova H, Guran R, Zitka O, Richtera L, Hegerova D, Heger Z, Moulick A, Adam V (2018) Novel vancomycin-peptide conjugate as potent antibacterial agent against vancomycin-resistant Staphylococcus aureus. Infect Drug Resist 11:1807–1817. https://doi.org/10.2147/IDR.S160975
Zhou Z, Peng S, Sui M, Chen S, Huang L, Xu H, Jiang T (2018) Multifunctional nanocomplex for surface-enhanced Raman scattering imaging and near-infrared photodynamic antimicrobial therapy of vancomycin-resistant bacteria. Colloids Surf B Biointerfaces 161:394–402. https://doi.org/10.1016/j.colsurfb.2017.11.005
Eichel V, Klein S, Bootsveld C, Frank U, Heeg K, Boutin S, Nurjadi D (2020) Challenges in interpretation of WGS and epidemiological data to investigate nosocomial transmission of vancomycin-resistant Enterococcus faecium in an endemic region: incorporation of patient movement network and admission screening. J Antimicrob Chemother 75(7):1716–1721. https://doi.org/10.1093/jac/dkaa122
Dos Santos EMP, Martins CCB, de Oliveira Santos JV, da Silva WRC, Silva SBC, Pelagio-Flores MA, Galembeck A, Cavalcanti IMF (2021) Silver nanoparticles-chitosan composites activity against resistant bacteria: tolerance and biofilm inhibition. J Nanopart Res 23(8):196. https://doi.org/10.1007/s11051-021-05314-1
Moreno-Valle B, Alatorre-Barajas JA, Gochi-Ponce Y, Alcántar-Zavala E, Rivera-Lugo YY, Montes-Ávila J, Trujillo-Navarrete B, Alonso-Núñez G, Reynoso-Soto EA, Ochoa-Terán A (2020) MWCNT-oxazolidinone conjugates with antibacterial activity. J Nanopart Res 22(11):315. https://doi.org/10.1007/s11051-020-05044-w
Wang R, Kim D, Yang M, Li X, Yoon J (2022) Phthalocyanine-assembled “one-for-two” nanoparticles for combined photodynamic-photothermal therapy of multidrug-resistant bacteria. ACS Appl Mater Interfaces 14(6):7609–7616. https://doi.org/10.1021/acsami.1c21891
ElZorkany HE, Youssef T, Mohamed MB, Amin RM (2019) Photothermal versus photodynamic treatment for the inactivation of the bacteria Escherichia coli and Bacillus cereus: an in vitro study. Photodiagnosis Photodyn Ther 27:317–326. https://doi.org/10.1016/j.pdpdt.2019.06.020
Shen T, Hu X, Liu Y, Zhang Y, Chen K, Xie S, Ke G, Song G, Zhang XB (2020) Specific core-satellite nanocarriers for enhanced intracellular ROS generation and synergistic photodynamic therapy. ACS Appl Mater Interfaces 12(5):5403–5412. https://doi.org/10.1021/acsami.9b16934
Zheng P, Fan M, Liu H, Zhang Y, Dai X, Li H, Zhou X, Hu S, Yang X, Jin Y, Yu N, Guo S, Zhang J, Liang XJ, Cheng K, Li Z (2021) Self-propelled and near-infrared-phototaxic photosynthetic bacteria as photothermal agents for hypoxia-targeted cancer therapy. ACS Nano 15(1):1100–1110. https://doi.org/10.1021/acsnano.0c08068
Bacali C, Carpa R, Buduru S, Moldovan ML, Baldea I, Constantin A, Moldovan M, Prodan D, Dascalu Rusu LM, Lucaciu O, Catoi F, Constantiniuc M, Badea M (2021) Association of graphene silver polymethyl methacrylate (PMMA) with photodynamic therapy for inactivation of halitosis responsible bacteria in denture wearers. Nanomaterials (Basel) 11(7):1643. https://doi.org/10.3390/nano11071643
Yougbare S, Mutalik C, Krisnawati DI, Kristanto H, Jazidie A, Nuh M, Cheng TM, Kuo TR (2020) Nanomaterials for the photothermal killing of bacteria. Nanomaterials (Basel) 10(6):1123. https://doi.org/10.3390/nano10061123
Chen Z, Tu Y, Zhang D, Liu C, Zhou Y, Li X, Wu X, Liu R (2020) A thermosensitive nanoplatform for photoacoustic imaging and NIR light triggered chemo-photothermal therapy. Biomater Sci 8(15):4299–4307. https://doi.org/10.1039/d0bm00810a
Yougbare S, Chou HL, Yang CH, Krisnawati DI, Jazidie A, Nuh M, Kuo TR (2021) Facet-dependent gold nanocrystals for effective photothermal killing of bacteria. J Hazard Mater 407:124617. https://doi.org/10.1016/j.jhazmat.2020.124617
Xing B, Jiang T, Bi W, Yang Y, Li L, Ma M, Chang CK, Xu B, Yeow EK (2011) Multifunctional divalent vancomycin: the fluorescent imaging and photodynamic antimicrobial properties for drug resistant bacteria. Chem Commun (Camb) 47(5):1601–1603. https://doi.org/10.1039/c0cc04434b
Jiao X, Zhang W, Zhang L, Cao Y, Xu Z, Kang Y, Xue P (2020) Rational design of oxygen deficient TiO2-x nanoparticles conjugated with chlorin e6 (Ce6) for photoacoustic imaging-guided photothermal/photodynamic dual therapy of cancer. Nanoscale 12(3):1707–1718. https://doi.org/10.1039/c9nr09423g
Zhang Z, Xu W, Kang M, Wen H, Guo H, Zhang P, Xi L, Li K, Wang L, Wang D, Tang BZ (2020) An all-round athlete on the track of phototheranostics: subtly regulating the balance between radiative and nonradiative decays for multimodal imaging-guided synergistic therapy. Adv Mater 32(36):e2003210. https://doi.org/10.1002/adma.202003210
Wang Y, Gong N, Li Y, Lu Q, Wang X, Li J (2020) Atomic-level nanorings (A-NRs) therapeutic agent for photoacoustic imaging and photothermal/photodynamic therapy of cancer. J Am Chem Soc 142(4):1735–1739. https://doi.org/10.1021/jacs.9b11553
Zhao X, Long S, Li M, Cao J, Li Y, Guo L, Sun W, Du J, Fan J, Peng X (2020) Oxygen-dependent regulation of excited-state deactivation process of rational photosensitizer for smart phototherapy. J Am Chem Soc 142(3):1510–1517. https://doi.org/10.1021/jacs.9b11800
Qi X, Jiang F, Zhou M, Zhang W, Jiang X (2021) Graphene oxide as a promising material in dentistry and tissue regeneration: a review. Smart Materials in Medicine 2:280–291. https://doi.org/10.1016/j.smaim.2021.08.001
Campbell E, Hasan MT, Pho C, Callaghan K, Akkaraju GR, Naumov AV (2019) Graphene oxide as a multifunctional platform for intracellular delivery, imaging, and cancer sensing. Sci Rep 9(1):416. https://doi.org/10.1038/s41598-018-36617-4
Han JL, Xia X, Tao Y, Yun H, Hou YN, Zhao CW, Luo Q, Cheng HY, Wang AJ (2016) Shielding membrane surface carboxyl groups by covalent-binding graphene oxide to improve anti-fouling property and the simultaneous promotion of flux. Water Res 102:619–628. https://doi.org/10.1016/j.watres.2016.06.032
Singh V, Kumar V, Kashyap S, Singh AV, Kishore V, Sitti M, Saxena PS, Srivastava A (2019) Graphene oxide synergistically enhances antibiotic efficacy in vancomycin-resistant Staphylococcus aureus. ACS Appl Bio Mater 2(3):1148–1157. https://doi.org/10.1021/acsabm.8b00757
Di Crescenzo A, Zara S, Di Nisio C, Ettorre V, Ventrella A, Zavan B, Di Profio P, Cataldi A, Fontana A (2019) Graphene oxide foils as an osteoinductive stem cell substrate. ACS Appl Bio Mater 2(4):1643–1651. https://doi.org/10.1021/acsabm.9b00041
Ma G, Qi J, Cui Q, Bao X, Gao D, Xing C (2020) Graphene oxide composite for selective recognition, capturing, photothermal killing of bacteria over mammalian cells. Polymers (Basel) 12(5):1116. https://doi.org/10.3390/polym12051116
Wu J, Li Z, Li Y, Pettitt A, Zhou F (2018) Photothermal effects of reduced graphene oxide on pancreatic cancer. Technol Cancer Res Treat 17:1533034618768637. https://doi.org/10.1177/1533034618768637
Wang H, Chen T, Wu S, Chu X, Yu R (2012) A novel biosensing strategy for screening G-quadruplex ligands based on graphene oxide sheets. Biosens Bioelectron 34(1):88–93. https://doi.org/10.1016/j.bios.2012.01.023
Wu S, Duan N, Ma X, Xia Y, Wang H, Wang Z, Zhang Q (2012) Multiplexed fluorescence resonance energy transfer aptasensor between upconversion nanoparticles and graphene oxide for the simultaneous determination of mycotoxins. Anal Chem 84(14):6263–6270. https://doi.org/10.1021/ac301534w
He X, Zhou Z, Han Z, Zeng Y, Chen X, Su J (2019) Mechanism of controlled release of vancomycin from crumpled graphene oxides. ACS Omega 4(7):12252–12258. https://doi.org/10.1021/acsomega.9b00873
Zhang Q, Li N, Goebl J, Lu Z, Yin Y (2011) A systematic study of the synthesis of silver nanoplates: is citrate a “magic” reagent? J Am Chem Soc 133(46):18931. https://doi.org/10.1021/ja2080345
Tian B, Wang C, Zhang S, Feng L, Liu Z (2011) Photothermally enhanced photodynamic therapy delivered by nano-graphene oxide. ACS Nano 5(9):7000–7009. https://doi.org/10.1021/nn201560b
Yin M, Yang M, Yan D, Yang L, Wan X, Xiao J, Yao Y, Luo J (2022) Surface-charge-switchable and size-transformable thermosensitive nanocomposites for chemo-photothermal eradication of bacterial biofilms in vitro and in vivo. ACS Appl Mater Interfaces 14(7):8847–8864. https://doi.org/10.1021/acsami.1c24229
Zhang C, Huang L, Sun DW, Pu H (2022) Interfacing metal-polyphenolic networks upon photothermal gold nanorods for triplex-evolved biocompatible bactericidal activity. J Hazard Mater 426:127824. https://doi.org/10.1016/j.jhazmat.2021.127824
Xu LQ, Liao YB, Li NN, Li YJ, Zhang JY, Wang YB, Hu XF, Li CM (2018) Vancomycin-assisted green synthesis of reduced graphene oxide for antimicrobial applications. J Colloid Interface Sci 514:733–739. https://doi.org/10.1016/j.jcis.2018.01.014
Mirzaie V, Ansari M, Nematollahi-Mahani SN, MoballeghNasery M, Karimi B, Eslaminejad T, Pourshojaei Y (2020) Nano-graphene oxide-supported APTES-spermine, as gene delivery system, for transfection of pEGFP-p53 into breast cancer cell lines. Drug Des Dev Ther 14:3087–3097. https://doi.org/10.2147/DDDT.S251005
Liu Z, Zhu Y, Liu X, Yeung KWK, Wu S (2017) Construction of poly (vinyl alcohol)/poly (lactide-glycolide acid)/vancomycin nanoparticles on titanium for enhancing the surface self-antibacterial activity and cytocompatibility. Colloids Surf, B 151:165–177. https://doi.org/10.1016/j.colsurfb.2016.12.016
Dinache A, Boni M, Alexandru T, Radu E, Stoicu A, Andrei IR, Staicu A, Liggieri L, Nastasa V, Pascu ML, Ferrari M (2015) Surface properties of vancomycin after interaction with laser beams. Colloids Surf, A 480:328–335
Anaya-Plaza E, Aljarilla A, Beaune G, Nonappa TJVI, de la Escosura A, Torres T, Kostiainen MA (2019) Phthalocyanine-virus nanofibers as heterogeneous catalysts for continuous-flow photo-oxidation processes. Adv Mater 31(39):e1902582. https://doi.org/10.1002/adma.201902582
Wan W, Triana CA, Lan J, Li J, Allen CS, Zhao Y, Iannuzzi M, Patzke GR (2020) Bifunctional single atom electrocatalysts: coordination-performance correlations and reaction pathways. ACS Nano 14(10):13279–13293. https://doi.org/10.1021/acsnano.0c05088
Zhang G, Liu B, Zhang Y, Li T, Chen W, Zhao W (2020) Study on the effects of a pi electron conjugated structure in binuclear metallophthalocyanines graphene-based oxygen reduction reaction catalysts. Nanomaterials 10(5):946. https://doi.org/10.3390/nano10050946
Acknowledgements
We thank the Natural Science Foundation of Shandong Province, China (ZR2020MC079, ZR2014BP004), National Natural Science Foundation of China (21505066), and a project of Shandong Province Higher Educational Science and Technology Program, China (J18KA265).
Author information
Authors and Affiliations
Contributions
Huijie Wang: Data curation, Writing an original draft. Xiaoli Yu: Methodology, Data curation, Formal analysis. Qixian Li: Methodology, Formal analysis. Jingru Zhu: Formal analysis, Data curation. Juan Ding: Data curation. Tingting Jiang: Supervision, Conceptualization, Methodology, Writing review and editing. All the authors reviewed and edited the manuscript and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, H., Yu, X., Li, Q. et al. Vancomycin modified graphene oxide as carriers to load phthalocyanine for synergistic phototherapy of vancomycin-resistant bacteria. J Nanopart Res 24, 234 (2022). https://doi.org/10.1007/s11051-022-05608-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-022-05608-y