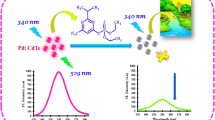
Abstract
Surface plasmons are one of the most important aspects of nano-photonics, which studies the features of incident electromagnetic light radiation with the metallic nanoparticles (NPs) when the size of NPs becomes smaller than the wavelength of the light. Organic dyes or fluorophores are generally used for the sensing purpose, but quantum dots (QDs) can also be utilized as an alternate for optical sensing. A novel detection system based on the photoluminescence (PL) enhancement of Ag-CdS QDs for the direct measurement of ammonia solution (NH4OH). The present work focuses on the fabrication of hybrid Ag-CdS QDs solution which behaves as an optical sensor for NH4OH. The synthesized samples were characterized by PL spectroscopy for their optical properties and transmission electron microscopy for their morphological studies. This is an effective method which exhibits the effect of concentration of NH4OH on the emission intensity of Ag-CdS QDs. Due to the presence of Ag NPs in the prepared samples of hybrid Ag-CdS QDs, the experimental results showed an enhancement in the emission intensity by adding NH4OH with varying concentration from 30 (0.03 M) to 870 mM (0.87 M). Strong enhancement in PL intensity is observed as we further increase the concentration of NH4OH from 1 to 4.5 M. The results presented here provide the rapid fabrication of an ultra-low-cost, simple and reproducible ammonia sensor to minimize the exposure to diseases by continuous monitoring of water quality.












Similar content being viewed by others
References
Chaudhuri RG, Paria S (2012) Core/shell nanoparticles: classes, properties, synthesis mechanisms, characterization, and applications. Chem Rev 112:2373–2433
Alivisatos AP (1996) Semiconductor clusters, nanocrystals, and quantum dots. Science 271:933–937
Murphy CJ, Coffer JL (2002) Quantum dots: a primer. Appl Spectrosc 56:16A-27A
Reid PJ, Fujimoto B, Gamelin DR (2014) A simple ZnO nanocrystal synthesis illustrating three-dimensional quantum confinement. J Chem Educ 91:280–282
Murray C, Norris DJ, Bawendi MG (1993) Synthesis and characterization of nearly monodisperse CdE (E= sulfur, selenium, tellurium) semiconductor nanocrystallites. J Am Chem Soc 115:8706–8715
Brus LE (1984) Electron-electron and electron-hole interactions in small semiconductor crystallites: the size dependence of the lowest excited electronic state. J Chem Phys 80:4403–4409
Roy MD, Herzing AA, Lacerda SHDP, Becker ML (2008) Emission-tunable microwave synthesis of highly luminescent water soluble CdSe/ZnS quantum dots. Chem Comm 18:2106–2108
Koch SW (1993) Semiconductor quantum dots 2: World Scientific
Jaiswal JK, Simon SM (2004) Potentials and pitfalls of fluorescent quantum dots for biological imaging. Trends Cell Biol. 14:497–504
Bruchez M, Moronne M, Gin P, Weiss S, Alivisatos AP (1998) Semiconductor nanocrystals as fluorescent biological labels. Science 281:2013–2016
Chan WCW, Nie SM (1998) Quantum dot bioconjugates for ultrasensitive as fluorescent biological labels. Science 281:2016–2018
Kramer IJ, Sargent EH (2014) The architecture of colloidal quantum dot solar cells: materials to devices. Chem Rev 114:863–882
Hikmet RA, Chin PT, Talapin DV, Weller H (2005) Polarized light emitting quantum rod diodes. Adv Mater 17:1436–1439
Kim DH, Lu N et al (2011) Epidermal electronics. Science 333:838–843
Zhai C, Zhang H, Du N, Chen B, Huang H, Wu Y, Yang D (2011) One-pot synthesis of biocompatible CdSe/CdS quantum dots and their applications as fluorescent biological labels. Nanoscale Res Lett 6:1–5
Zhang Y, Li Y, Yan XP (2009) Aqueous layer-by-layer epitaxy of type-II CdTe/CdSe quantum dots with near-infrared fluorescence for bioimaging applications. Small 5:185–189
Chan WC, Maxwell DJ, Gao X, Bailey RE, Han M, Nie S (2002) Luminescent quantum dots for multiplexed biological detection and imaging. Curr Opin Biotechnol 13:40–46
Hsu YJ, Lu SY (2008) Dopant-Induced Formation of Branched CdS Nanocrystals. Small 4:951–955
Haldar KK, Sinha G, Lahtinen J, Patra A (2012) Hybrid colloidal Au-CdSe pentapod heterostructures synthesis and their photocatalytic properties. ACS Appl Mater Interfaces 4:6266–6272
Silvi S, Credi A (2015) Luminescent sensors based on quantum dot–molecule conjugates. Chem Soc Rev 44:4275–4289
Dutta RK, Kumar A (2016) Highly sensitive and selective method for detecting ultratrace levels of aqueous uranyl ions by strongly photoluminescent-responsive amine-modified cadmium sulfide quantum dots. Anal Chem 88:9071–9078
Warneck P (1999) Chemistry of the natural atmosphere. Elsevier
Huff L, Delos C, Gallagher K, Beaman J (2013) Aquatic life ambient water quality criteria for ammonia-freshwater. U.S. Environmental Protection Agency, 10
Li T, Panther J, Qiu Y et al (2017) Gas-permeable membrane-based conductivity probe capable of in situ real-time monitoring of ammonia in aquatic environments. Environ Sci Technol 51:13265–13273
Huang F, Lan Y (2015) Aqueous synthesis of water-soluble l-cysteine-modified cadmium sulfide doped with silver ion/zinc sulfide nanocrystals. Spectrosc Lett 48(3):159–162
Lin L, Wen Y, Liang Y, Zhang N, Xiao D (2013) Aqueous synthesis of Ag+ doped CdS quantum dots and its application in H 2 O 2 sensing. Anal Methods 5(2):457–464
Marimuthu S, Rahuman AA, Rajakumar G et al (2011) Evaluation of green synthesized silver nanoparticles against parasites. Parasitol Res 108:1541–1549
Senthilkumar SR, Sivakumar T (2014) Green tea (Camellia Sinensis) mediated synthesis of zinc oxide (ZnO) nanoparticles and studies on their antimicrobial activities. Int J Pharm Pharm Sci 6:6
Mayer KM, Hafner JH (2011) Localized surface plasmon resonance sensors. Chem Rev 111:3828–3857
Harry ST, Adekanmbi MA (2020) Confinement energy of quantum dots and the brus equation. Int J Res Granthaalayah 8:318–323
Yu WW, Qu L, Guo W, Peng X (2003) Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem Mater 15:2854–2860
Khurgin JB, Sun G, Soref RA (2007) Enhancement of luminescence efficiency using surface plasmon polaritons: figures of merit. JOSA B 24:1968–1980
Mohammadi A, Kaminski F, Sandoghdar V, Agio M (2009) Spheroidal nanoparticles as nanoantennas for fluorescence enhancement. Int J Nanotechnol 6:902–914
Acknowledgements
We are thankful to the Director, National institute of Technology, Kurukshetra, for all the facilities and also thankful to CSIR, New Delhi, for giving funding in the form of project (03(1440)/18/EMR-II).
Funding
The research was financially supported by the Director, National institute of Technology, Kurukshetra, and CSIR, New Delhi.
Author information
Authors and Affiliations
Contributions
Both authors have the same contributions.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khurana, K., Jaggi, N. An aqueous ammonia detection by hybrid Ag-CdS quantum dots. J Nanopart Res 24, 216 (2022). https://doi.org/10.1007/s11051-022-05600-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-022-05600-6