Abstract
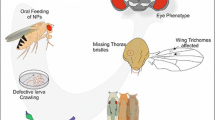
Tungsten oxide nanoparticles (WO3 NPs) have now been employed by various products including electronics, smart screens, gas-biosensors, water purifiers, disinfectants, and biomedical applications. Despite this wide-ranging adoption, little research has investigated their potential endpoint biomarkers in different in vivo models. We therefore propose the use of Drosophila melanogaster as a testing model in assessing genotoxic risks of exposure to WO3 NPs. Our study examined toxicity, phenotypic alterations, locomotor behavior (climbing assay), intracellular oxidative stress (ROS), DNA damage (Comet assay), and somatic recombination (wing spot assay) in Drosophila melanogaster after exposure to WO3 NPs (43.71 ± 1.59 nm) and microparticulated (MPs) of WO3. Drosophila larvae were exposed to the test materials via ingestion at doses ranging between 1 and 10 mM, and two greatest doses of NPs (5 and 10 mM) were found to cause mutagenic/recombinogenic effects, while the MPs caused no effects. Wing-spot assay detected genotoxic activity of NPs mostly through somatic recombination, and Comet assay showed DNA damage after exposure to NPs at certain doses (1, 2.5, 5, and 10 mM). Other observations included ROS generation in hemocytes, phenotypic alterations in the mouths and wings of adult flies, and impaired locomotor behavior. This is the first research to report genotoxic evidence on the impact of WO3 exposure in Drosophila larvae, highlighting the significance of this model organism in exploring the potential biological impact of nanoparticles and MPs of WO3. The results of our in vivo testing should make a vital contribution to the existing database on the genotoxicity of WO3 NPs.








Similar content being viewed by others
References
Hasan S (2015) A review on nanoparticles: their synthesis and types biosynthesis: mechanism. Res J Recent Sci 4:9–11
Bradfield SJ, Kumar P, White JC, Ebbs SD (2017) Zinc, copper, or cerium accumulation from metal oxide nanoparticles or ions in sweet potato: yield effects and projected dietary intake from consumption. Plant Physiol Biochem 110:128–137
Nowack B, Bucheli TD (2007) Occurrence, behavior and effects of nanoparticles in the environment. Environ Pollut 150:5–22
McShan D, Ray PC, Yu H (2014) Molecular toxicity mechanism of nanosilver. J Food Drug Anal 22:116–127
Alaraby M, Demir E, Domenech J, Velázquez A, Hernández A, Marcos R (2020) In vivo evaluation of the toxic and genotoxic effects of exposure to cobalt nanoparticles using Drosophila melanogaster. Environ Sci Nano 7:610–622
Demir E (2021) Adverse biological effects of ingested polystyrene microplastics using Drosophila melanogaster as a model in vivo organism. J Toxicol Environ Health Part A 84:649–660
Ong C, Yung LYL, Cai Y, Bay BH, Baeg GH (2015) Drosophila melanogaster as a model organism to study nanotoxicity. Nanotoxicology 9:396–403
Chinde S, Grover P (2017) Toxicological assessment of nano and micron-sized tungsten oxide after 28 days repeated oral administration to Wistar rats. Mutat Res Genet Toxicol Environ Mutagen 819:1–13
Erik L, Wolf-Dieter S (1999) Tungsten: properties, chemistry, technology of the element, alloys, and chemical compounds. Kluwer Academic, USA
Zhou G, Hou Y, Liu L, Liu H, Liu C, Liu J, Qiao H, Liu W, Fan Y, Shen S, Rong L (2012) Preparation and characterization of NiW-nHA composite catalyst for hydrocracking. Nanoscale 4:7698–7703
Cong S, Geng F, Zhao Z (2016) Tungsten oxide materials for optoelectronic applications. Adv Mater 28:10518–10528
Granqvist CG (2014) Electrochromics for smart windows: oxide based thin films and devices. Thin Solid Films 564:1–38
Hu L, Hu P, Chen Y, Lin Z, Qiu C (2018) Synthesis and gas-sensing property of highly self-assembled tungsten oxide nanosheets. Front Chem 6:452
Chen X, Zhou Y, Liu Q, Li Z, Liu J, Zou Z (2012) Ultrathin, single-crystal wo3 nanosheets by two-dimensional oriented attachment toward enhanced photocatalystic reduction of CO2 into hydrocarbon fuels under visible light. ACS Appl Mater Interfaces 4:3372–3377
Wang P, Huang B, Qin X, Zhang X, Dai Y, Whangbo MH (2009) Ag/AgBr/WO3·H2O: visible-light photocatalyst for bacteria destruction. Inorg Chem 48:10697–10702
Ahmed S, Hassan IAI, Roy H, Marken F (2013) Photoelectrochemical transients for chlorine/hypochlorite formation at “Roll-On” nano-WO3 film electrodes. J Phys Chem C 117:7005–7012
Hasegawa G, Shimonaka M, Ishihara Y (2012) Differential genotoxicity of chemical properties and particle size of rare metal and metal oxide nanoparticles. J Appl Toxicol 32:72–80
Turkez H, Sonmez E, Turkez O, Mokhtar YI, Stefano AD, Turgut G (2014) The risk evaluation of tungsten oxide nanoparticles in cultured rat liver cells for its safe applications in nanotechnology. Braz Arch Biol Technol 57:532–541
Ivask A, Titma T, Visnapuu M, Vija H, Kakinen A, Sihtmae M, Kahru A (2015) Toxicity of 11 metal oxide nanoparticles to three mammalian cell types in vitro. Curr Top Med Chem 5:1914–1929
Chinde S, Dumala N, Rahman MF, Kamal SSK, Kumari SI, Mahboob M, Grover P (2017) Toxicological assessment of tungsten oxide nanoparticles in rats after acute oral exposure. Environ Sci Pollut Res 24:13576–13593
Hassanvand A, Zare MH, Shams A, Nickfarjam A, Shabani M, Rahavi H (2019) Investigation of the effect of radiosensitization of tungsten oxide nanoparticles on AGS cell line of human stomach cancer in megavoltage photons radiation. J Nanostructures 9:563–578
Akbaba BG, Turkez H, Sonmez E, Akbaba U, Aydın E, Tatar A, Turgut G, Cerig S (2016) In vitro genotoxicity evaluation of tungsten (VI) oxide nanopowder using human lymphocytes. Biomed Res 27:229–234
Turkez H, Cakmak B, Celik K (2013) Evaluation of the potential in vivo genotoxicity of tungsten (VI) oxide nanopowder for human health. Key Eng Mater 543:89–92
Prajapati MV, Adebolu OO, Morrow BM, Cerreta JM (2017) Evaluation of pulmonary response to inhaled tungsten (iv) oxide nanoparticles in golden syrian hamsters. Exper Biol Med 242:29–44
Areecheewakul S, Adamcakova-Dodd A, Givens BE, Steines BR, Wang Y, Meyerholz DK, Parizek NJ, Altmaier R, Haque E, O’Shausghnessy PT, Salem AK, Thorne PS (2020) Toxicity assessment of metal oxide nanomaterials using in vitro screening and murine acute inhalation studies. NanoImpact 18:100214
Mao L, Zheng L, You H, Ullah MW, Cheng H, Guo Q, Li R (2021) A comparison of hepatotoxicity induced by different lengths of tungsten trioxide nanorods and the protective effects of melatonin in BALB/c mice. Environ Sci Pollut Res 28:40793–40807
Contreras EQ, Cho M, Zhu H, Puppala HL, Escalera G, Zhong W, Colvin VL (2012) Toxicity of quantum dots and cadmium salt to Caenorhabditis elegans after multigenerational exposure. Environ Sci Technol 47:1148–1154
Hunt PR, Marquis BJ, Tyner KM, Conklin S, Olejnik N, Nelson BC, Sprando RL (2013) Nanosilver suppresses growth and induces oxidative damage to DNA in Caenorhabditis elegans. J Appl Toxicol 33:1131–1142
Chatterjee N, Eom HJ, Choi J (2014) Effects of silver nanoparticles on oxidative DNA damage-repair as a function of p38 MAPK status: a comparative approach using human Jurkat T cells and the nematode Caenorhabditis elegans. Environ Mol Mutagen 55:122–133
Chatterjee N, Yang J, Kim HM (2014) Potential toxicity of differential functionalized multiwalled carbon nanotubes (MWCNT) in human cell line (BEAS2B) and Caenorhabditis elegans. J Toxicol Environ Health Part A 77:1399–1408
Pappus SA, Mishra M (2018) A Drosophila model to decipher the toxicity of nanoparticles taken through oral routes. Adv Exp Med Biol 1048:311–322
Gao M, Zhang Z, Lv M, Song W, Lv Y (2018) Toxic effects of nanomaterial-adsorbed cadmium on Daphnia magna. Ecotoxicol Environ Saf 148:261–268
Shariati F, Poordeljoo T, Zanjanchi P (2020) The acute toxicity of SiO2 and Fe3O4 nano-particles on Daphnia magna. SILICON 12:2941–2946
Pappus SA, Ekka B, Sahu S, Sabat D, Dash P, Mishra M (2017) A toxicity assessment of hydroxyapatite nanoparticles on development and behaviour of Drosophila melanogaster. J Nanoparticle Res 19(4):136
Anand AS, Gahlot U, Prasad DN, Amitabh Kohli E (2019) Aluminum oxide nanoparticles mediated toxicity, loss of appendages in progeny of Drosophila melanogaster on chronic exposure. Nanotoxicology 13:977–989
Demir E (2020) An in vivo study of nanorod, nanosphere, and nanowire forms of titanium dioxide using Drosophila melanogaster: toxicity, cellular uptake, oxidative stress, and DNA damage. J Toxicol Environ Health Part A 83:456–469
Mendoza RP, Brown JM (2019) Engineered nanomaterials and oxidative stress: current understanding and future challenges. Curr Opin Toxicol 13:74–80
Dan P, Sundararajan V, Ganeshkumar H, Gnanabarathi B, Subramanian AK, Venkatasubu GD, Ichihara SS (2019) Evaluation of hydroxyapatite nanoparticles-induced in vivo toxicity in Drosophila melanogaster. Appl Surf Sci 484:568–577
Mishra M, Panda M (2021) Reactive oxygen species: The root cause of nanoparticle-induced toxicity in Drosophila melanogaster. Free Radic Res 55:919–935
Reiter LT, Potocki L, Chien S, Gribskov M, Bier E (2001) A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res 88:1114–1125
Demir E, Turna Demir F, Marcos R (2022) Drosophila as a suitable in vivo model in the safety assessment of nanomaterials. Adv Exp Med Biol 1357:275–301
Wolf MJ, Amrein H, Izatt JA, Choma MA, Reedy MC, Rockman HA (2006) From the cover: Drosophila as a model for the identification of genes causing adult human heart disease. Proc Natl Acad Sci 103:1394–1399
Bier E (2005) Drosophila, the golden bug, emerges as a tool for human genetics. Nat Rev Genet 6:9–23
Gonzalez C (2013) Drosophila melanogaster: a model and a tool to investigate malignancy and identify new therapeutics. Nat Rev Cancer 13:172–183
Latouche M, Lasbleiz C, Martin E, Monnier V, Debeir T, Mouatt-Prigent A, Muriel MP, Morel L, Ruberg M, Brice A, Stevanin G, Tricoire H (2007) A conditional pan neuronal drosophila model of spinocerebellar ataxia 7 with a reversible adult phenotype suitable for identifying modifier genes. J Neurosci Res 27:2483–2492
Bilen J, Bonini NM (2005) Drosophila as a model for human neurodegenerative disease. Annu Rev Genet 39:153–171
Moloney A, Sattelle DB, Lomas DA, Crowther DC (2010) Alzheimer’s disease: insightsFrom Drosophila melanogaster models. Trends Biochem Sci 35:228–235
Ng CT, Ong CN, Yu LE, Bay BH, Baeg GH (2019) Toxicity study of zinc oxide nanoparticles in cell culture and in Drosophila melanogaster. J Vis Exp 151:e59510
Flecknell P (2002) Replacement, reduction and refinement. Altex 19:73–78
Jennings BH (2011) Drosophila-a versatile model in biology & medicine. Mater Today 14:190–195
Rand MD (2010) Drosophotoxicology: the growing potential for Drosophila in neurotoxicology. Neurotoxicol Teratol 32:74–83
Rand MD, Vorojeikina D, Peppriell A, Gunderson J, Prince LM (2019) Drosophotoxicology: elucidating kinetic and dynamic pathways of methylmercury toxicity in a Drosophila model. Front Genet 10:666
Chifiriuc MC, Ratiu AC, Popa M, Ecovoiu AA (2016) Drosophotoxicology: an emerging research area for assessing nanoparticles interaction with living organisms. Int J Mol Sci 17:36
Benford DJ, Hanley AB, Bottrill K, Oehlschlager S, Balls M, Branca F, Castegnaro JJ, Descotes J, Hemminiki K, Lindsay D, Schilter B (2000) Biomarkers as predictive tools in toxicity testing. ATLA 28:119–131
Rajak P, Dutta M, Roy S (2015) Altered differential hemocyte count in 3rd instar larvae of Drosophila melanogaster as a response to chronic exposure of acephate. Interdiscip Toxicol 8:84–88
Festing MFW, Baumans V, Combes DR, Hadler M, Hendriksen FM, Howard BR, Lovell DP, Moore GJ, Overend P, Wilson MS (1998) Reducing the use of laboratory animals in biomedical research: problems and possible solutions. Altern Lab Anim 26:283–301
Demir E, Marcos R (2018) Antigenotoxic potential of boron nitride nanotubes. Nanotoxicology 12:868–884
Demir E (2022) Mechanisms and biological impacts of graphene and multi-walled carbon nanotubes on Drosophila melanogaster: oxidative stress, genotoxic damage, phenotypic variations, locomotor behavior, parasitoid resistance, and cellular immune response. J Appl Toxicol 42:450–474
Demir E, Turna F, Aksakal S, Kaya B, Marcos R (2014) Genotoxicity of different sweeteners in Drosophila. Fresenius Environ Bull 23:3426–3432
Demir E, Marcos R (2017) Assessing the genotoxic effects of two lipid peroxidation products (4-oxo-2-nonenal and 4-hydroxy-hexenal) in haemocytes and midgut cells of Drosophila melanogaster larvae. Food Chem Toxicol 105:1–7
Nanogenotox (2011) http://www.nanogenotox.eu/files/PDF/Deliverables/nanogenotox%20deliverable%203_wp4_%20dispersion%20protocol.pdf
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Pendleton RG, Parvez F, Sayed M, Hillman R (2002) Effects of pharmacological agents upon a transgenic model of Parkinson’s disease in Drosophila melanogaster. J Pharmacol Exp Ther 300:91–96
Martinez VG, Javadi CS, Ngo E, Ngo L, Lagow RD, Zhang B (2007) Age-related changes in climbing behavior and neural circuit physiology in Drosophila. Dev Neurobiol 67:778–791
Anand AS, Prasad DN, Singh SB, Kohli E (2017) Chronic exposure of zinc oxide nanoparticles causes deviant phenotype in Drosophila melanogaster. J Hazard Mater 327:180–186
Priyadarsini S, Sahoo SK, Sahu S, Mukherjee S, Hota G, Mishra M (2019) Oral administration of graphene oxide nano-sheets induces oxidative stress, genotoxicity, and behavioral teratogenicity in Drosophila melanogaster. Environ Sci Pollut Res 26:19560–19574
Mishra M, Sabat D, Ekka B, Sahu S, Unnikannan P, Dash P (2017) Oral intake of zirconia nanoparticle alters neuronal development and behaviour of Drosophila melanogaster. J Nanoparticle Res 19:282
Sood K, Kaur J, Singh H, Arya SK, Khatri M (2019) Comparative toxicity evaluation of graphene oxide (GO) and zinc oxide (ZnO) nanoparticles on Drosophila melanogaster. Toxicol Rep 6:768–781
Graf U, Würgler FE, Katz AJ, Frei H, Juan H, Hall CB, Kale PG (1984) Somatic mutation and recombination test in Drosophila melanogaster. Environ Mol Mutagen 6:153–188
Lindsley DL, Zimm GG (1992) The genome of Drosophila melanogaster. Academic Press, San Diego, CA
Turna F, Aksakal S, Demir E, Kaya B (2014) Antigenotoxic effects of Resveratrol in somatic cells of Drosophila melanogaster. Fresenius Environ Bull 23:2116–2125
Irving P, Ubeda JM, Doucet D, Troxler L, Lagueux M, Zachary D, Hoffmann JA, Hetru C, Meister M (2005) New insights into Drosophila larval haemocyte functions through genome-wide analysis. Cell Microbiol 7:335–350
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Ghosh M, Manivannan J, Sinha S, Chakraborty A, Mallick SK, Bandyopadhyay M, Mukherjee A (2012) in vitro and in vivo genotoxicity of silver nanoparticles. Mutat Res 749:60–69
Tice RR, Andrews PW, Singh N (1990) The single cell gel assay. A sensitive technique for evaluating intercellular differences in DNA damage and repair. B.M. Sutherland, A.D. Wordhead (Eds.), DNA damage and repair in human tissues, Plenum, New York, NY (1990), pp. 291–302
Mukhopadhyay I, Chowdhuri DK, Bajpayee M, Dhawan A (2004) Evaluation of in vivo genotoxicity of cypermethrin in Drosophila melanogaster using the alkaline comet assay. Mutagenesis 19:85–90
Siddique HR, Chowdhuri DK, Saxena DK, Dhawan A (2005) Validation of Drosophila melanogaster as an in vivo model for genotoxicity assessment using modified alkaline comet assay. Mutagenesis 20:285–290
Końca K, Lankoff A, Banasik A, Lisowska H, Kuszewski T, Góźdź S, Koza Z, Wojcik A (2003) A cross-platform public domain pc image-analysis program for the comet assay. Mutat Res-Genet Toxicol Environ Mutagen 534:15–20
Turna Demir F, Yavuz M (2020) Heavy metal accumulation and genotoxic effects in levant vole (Microtus guentheri) collected from contaminated areas due to mining activities. Environ Pollut 256:113378
Kastenbaum MA, Bowman KO (1970) Tables for determining the statistical significance of mutation frequencies. Mutat Res 9:527–549
Frei H, Würgler FE (1995) Optimal experimental design and sample size for the statistical evaluation of data from somatic mutation and recombination tests (SMART) in Drosophila. Mutat Res 334:247–225
Frei H, Würgler FE (1988) Statistical methods to decide whether mutagenicity test data from Drosophila assays indicate a positive, negative, or inconclusive results. Mutat Res 203:297–308
Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, Khorasani S (2018) Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 10:57
Demir E, Vales G, Kaya B, Creus A, Marcos R (2011) Genotoxic analysis of silver nanoparticles in Drosophila. Nanotoxicology 5:417–424
Turna Demir F (2022) In vivo effects of 1,4-dioxane on genotoxic parameters and behavioral alterations in Drosophila melanogaster. J Toxicol Environ Health Part A 85:414–430
Karlsson HL, Gustafsson J, Cronholm P, Möller L (2009) Size-dependent toxicity of metal oxide particles-a comparison between nano-and micrometer size. Toxicol Lett 188:112–118
Arnold M, Badireddy A, Wiesner M, Di Giulio R, Meyer J (2013) Cerium oxide nanoparticles are more toxic than equimolar bulk cerium oxide in Caenorhabditis elegans. Arch Environ Contam Toxicol 65:224–233
Vales G, Demir E, Kaya B, Creus A, Marcos R (2013) Genotoxicity of cobalt nanoparticles and ions in Drosophila. Nanotoxicology 7:462–468
Demir E, Aksakal S, Turna F, Kaya B, Marcos R (2015) In vivo genotoxic effects of four different nano-sizes forms of silica nanoparticles in Drosophila melanogaster. J Hazard Mater 283:260–266
AshaRani PV, Mun GLK, Hande MP, Valiyaveettil S (2008) Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 3:279–290
Singh N, Manshian B, Jenkins GJ, Griffiths SM, Williams PM, Maffeis TG, Wright CJ, Doak SH (2009) Nanogenotoxicology: the DNA damaging potential of engineered nanomaterials. Biomaterials 30:3891–3914
Klien K, Godnić-Cvar J (2012) Genotoxicity of metal nanoparticles: focus on in vivo studies. Arh Hig Rada Toksikol 63:133–145
Magdolenova Z, Collins A, Kumar A, Dhawan A, Stone V, Dusinska M (2014) Mechanisms of genotoxicity. a review of in vitro and in vivo studies with engineered nanoparticles. Nanotoxicology 8:233–278
Demir E, Turna F, Burgucu D, Kılıç Z, Burunkaya E, Kesmez Ö, Kaya B (2013) Genotoxicity of different nano-sizes and ions of silica nanoparticles. Fresenius Environ Bull 22:2901–2909
Domenech J, Hernández A, Demir E, Marcos R, Cortés C (2020) Interactions of graphene oxide and graphene nanoplatelets with the in vitro caco-2/ht29 model of intestinal barrier. Sci Rep 10:1–15
Carmona ER, Guecheva TN, Creus A, Marcos R (2011) Proposal of an in vivo comet assay using haemocytes of Drosophila melanogaster. Environ Mol Mutagen 52:165–169
Gaivao I, Sierra LM (2014) Drosophila comet assay: insights, uses, and future perspectives. Front Genet 5:304
Alaraby M, Annangi B, Marcos R, Hernández A (2016) Drosophila melanogaster as a suitable in vivo model to determine potential side effects of nanomaterials: a review. J Toxicol Environ Health B Crit Rev 19:65–104
Wu X, Cobbina SJ, Mao G, Xu H, Zhang Z, Yang L (2016) A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut Res 23:8244–8259
Strawn ET, Cohen CA, Rzigalinski BA (2006) Cerium oxide nanoparticles increase lifespan and protect against free radical-mediated toxicity. FASEB J 20:A1356–A1356
Baeg E, Sooklert K, Sereemaspun A (2018) Copper oxide nanoparticles cause a dose-dependent toxicity via inducing reactive oxygen species in Drosophila. Nanomaterials 8:824
Paithankar JG, Kushalan S, Nijil S, Hegde S, Kini S, Sharma A (2022) Systematic toxicity assessment of cdte quantum dots in Drosophila melanogaster. Chemosphere 295:133836
Cui Y, Gong X, Duan Y, Li N, Hu R, Liu H, Hong F (2010) Hepatocyte apoptosis and its molecular mechanisms in mice caused by titanium dioxide nanoparticles. J Hazard Mater 183:874–880
Ng CT, Yong LQ, Hande MP, Ong CN, Yu LE, Bay BH, Baeg GH (2017) Zinc Oxide nanoparticles exhibit cytotoxicity and genotoxicity through oxidative stress responses in human lung fibroblasts and Drosophila melanogaster. Int J Nanomed 12:1621
Author information
Authors and Affiliations
Contributions
FTD and ED contributed to the conception, experimental design, experimental performance, data analysis, and writing original manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Turna Demir, F., Demir, E. Novel insights into acute/chronic genotoxic impact of exposure to tungsten oxide nanoparticles on Drosophila melanogaster. J Nanopart Res 24, 215 (2022). https://doi.org/10.1007/s11051-022-05593-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-022-05593-2